Naperiglipron Trial Terminations Highlight Shifts in Oral GLP-1 Market
Eli Lilly stopped two Phase 2 trials of naperiglipron after minimal enrollment, citing strategic reasons. Development continues in obesity, but structural similarity to Pfizer’s failed compounds raises safety scrutiny.
As of September 1, 2025, Eli Lilly has terminated two Phase 2 studies of naperiglipron (LY3549492), its next-generation oral GLP-1 receptor agonist, citing “strategic business reasons.” Each study had enrolled only one participant before being shut down. Despite this, Lilly continues development, with an active Phase 2 obesity trial (NCT06683508) and early-phase studies in Japan and China.
Clinical Trial Status (as of Aug 31, 2025)
|
Trial ID |
Description |
Status |
Key Details |
|
NCT07030868 |
Phase 2 in obesity/overweight + T2D (under master protocol
W8M-MC-CWMM) |
Terminated (strategic business reasons) |
Enrolled 1 participant; ran June–July 2025. |
|
NCT07085468 |
Phase 2 safety/tolerability in healthy older adults (BMI
22–25) |
Terminated (strategic business reasons) |
Enrolled 1 participant; ran July–Aug 2025. |
|
NCT06683508 |
Phase 2 obesity/overweight (under same master protocol) |
Active, not recruiting |
Duration ~1 year; results expected 2026. |
|
NCT06869018 / jRCT2071240136 |
Phase 1 multiple-ascending dose in Japanese T2D + healthy |
Recruiting |
Started May 2025; ~25-week duration. |
|
NCT07073170 |
Phase 1 multiple-dose escalation in Chinese T2D
participants |
Not yet recruiting |
Planned start Aug 2025; est. completion June 2026. |
🔬 Context and Outlook
- Structural safety overhang: Naperiglipron shares a benzimidazole-based GLP-1 agonist scaffold with Pfizer’s danuglipron (terminated Apr 2025 for liver injury) and lotiglipron (halted earlier for ALT elevations).
Figure: Comparative structures of naperiglipron, danuglipron, lotiglipron, and orforglipron
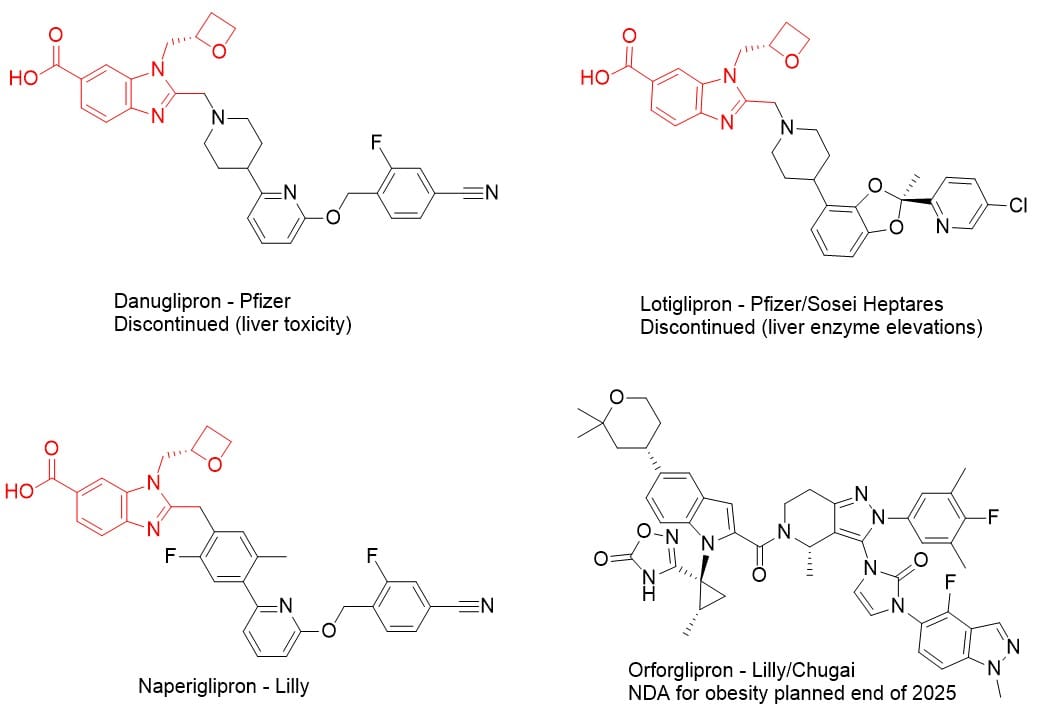
- Pipeline positioning: Orforglipron remains Lilly’s lead oral GLP-1, with Phase 3 obesity readouts showing ~12% weight loss and an FDA filing expected by end-2025. Naperiglipron was pitched as a “higher-efficacy next-gen” option, but its future now hinges on overcoming the scaffold’s baggage. Analysts (e.g., BMO, June 2025) have pointed to Lilly’s persistence as a bullish sign despite these risks. However, as highlighted in SinoDrugWatch, the rise of Chinese oral GLP-1 candidates and their strategic partnerships may influence global market dynamics and investor perspectives."
- Market competition: Novo Nordisk’s oral semaglutide (Wegovy pill) and oral amycretin are advancing, intensifying pressure on Lilly’s oral GLP-1 pipeline. Naperiglipron could have filled a potency niche, but its clinical viability is now cloudier.
Impact on the Oral GLP-1 Market
The termination of naperiglipron, alongside Pfizer’s danuglipron failure, highlights heightened scrutiny on small-molecule benzimidazole scaffolds. Peptide-based oral GLP-1s (oral semaglutide) and differentiated chemotypes like orforglipron now have a regulatory and competitive edge.
Ongoing programs will likely require extra liver-safety monitoring and longer follow-up, raising development costs. Lilly’s focus on orforglipron illustrates a broader trend of portfolio prioritization toward lower-risk, higher-confidence candidates.
Fewer global winners are expected, with incumbents dominating and other players competing on price, convenience, or niche indications. Chinese developers with differentiated chemotypes could gain local market share, but global adoption depends on convincing safety and efficacy data.
✅ Bottom Line
Lilly has pulled two early Phase 2 trials of naperiglipron after enrolling just one participant each, citing business strategy. The core obesity trial (NCT06683508) is still running, alongside Phase 1 work in Japan and China, but the program faces heightened scrutiny given its structural similarity to Pfizer’s failed oral GLP-1s.
Sources
- NCT07030868 — Phase 2 trial of naperiglipron in obesity/overweight with type 2 diabetes (terminated). ClinicalTrials.gov/study/NCT07030868
- NCT07085468 — Phase 2 safety/tolerability trial in healthy older adults (terminated). ClinicalTrials.gov/study/NCT07085468
- NCT06683508 — Phase 2 obesity/overweight trial (active, not recruiting). ClinicalTrials.gov/study/NCT06683508
- NCT06869018 — Phase 1 multiple-ascending dose study in Japanese participants (recruiting). ClinicalTrials.gov/study/NCT06869018
- NCT07073170 — Phase 1 multiple-dose escalation in Chinese participants with type 2 diabetes (not yet recruiting). ClinicalTrials.gov/study/NCT07073170
- jRCT2071240136 — Phase 1 multiple-ascending dose study in Japanese participants with type 2 diabetes and healthy volunteers (recruiting). jRCT.mhlw.go.jp/latest-detail/jRCT2071240136
- China’s Oral GLP-1 Surge — Rising amid global setbacks and intensifying competition. SinodrugWatch.com: https://www.sinodrugwatch.com/chinas-oral-glp-1-surge-rising-amid-global-setbacks-and-intensifying-competition