Braveheart Bio Takes HRS-1893 (BHB-1893) Global: Structure, Patents & Race vs Aficamten and Mavacamten
Hengrui’s HRS-1893, licensed to Braveheart Bio (BHB-1893), advances in oHCM (Phase III, NCT07021976) and nHCM (Phase II), with global expansion planned for 2026. Review covers structure, patents, PK, and clinical data.
Updated Nov 5, 2025: Included Braveheart Bio’s $185M Series A launch and global rights to HRS-1893 (now BHB-1893), plus additional structure, patent, and PK comparison details.
On September 5, 2025, Jiangsu Hengrui licensed HRS-1893 (now renamed BHB-1893) — a Phase III cardiac myosin inhibitor for obstructive hypertrophic cardiomyopathy (oHCM) — to Braveheart Bio, a newly launched biotech focused on genetically defined cardiovascular disease. The $1.088B global deal (ex-China) coincides with Braveheart’s $185M Series A financing led by Forbion, OrbiMed, and RA Capital, marking one of 2025’s largest cardiovascular start-ups. Braveheart will advance BHB-1893 globally to challenge BMS’s mavacamten (Camzyos) and Cytokinetics’ aficamten (CK-274) in the $2B+ HCM market, with Phase III (NCT07021976) already underway.
Following our detailed review of aficamten and its Phase III insights versus mavacamten, this review unpacks HRS-1893’s structural structure and patent strategy, preclinical PK profile versus mavacamten, Phase I ESC 2025 results, and ongoing Phase III trial (NCT07021976)—defining how a China-originated myosin inhibitor is entering the global race.
BHB-1893 (HRS-1893) structure & Patent Strategy
Hengrui patents (WO2022105852A1 and CN117088854A) define HRS-1893 (Braveheart code BHB-1893) as (S)-6-((1-(2-fluoro-5-methylphenyl)ethyl)amino)-3-(tetrahydro-2H-pyran-4-yl)-1,3,5-triazine-2,4(1H,3H)-dione (Chemical structures of HRS-1893/BHB-1893, mavacamten, and aficamten are shown in the figure below). Its triazine dione core differs from mavacamten’s pyrimidine-2,4-dione (US9585883, USRE50050; exp. June 2034). Key features: tetrahydropyran at position 3 and a chiral (S)-aryl ethylamine at position 6 for allosteric myosin binding.
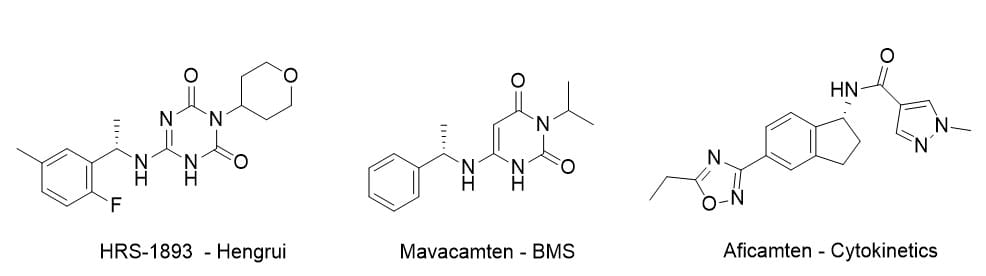
Preclinical PK Comparison: HRS-1893 (BHB-1893) vs. Mavacamten
(Data from WO2022105852A1 & CN117088854A)
|
Species |
Compound |
Route |
Dose (mg/kg) |
T₁/₂ (h) |
AUC (ng·h/mL) |
Notes |
|
SD Rat (14-day) |
HRS-1893 |
Oral |
5–30 |
4.8–6.9 |
30k–139k |
Stable, minimal accumulation |
|
SD Rat (14-day) |
Mavacamten |
Oral |
0.5–3 |
13–46.5 |
0.5k–16k |
Accumulates over 14 days |
|
Beagle Dog |
HRS-1893 |
Oral |
2 |
7.5 |
3,102 |
Rapid absorption, short half-life |
|
Beagle Dog |
Mavacamten |
Oral |
2 |
38.9 |
8,179 |
Long half-life, accumulates |
|
Monkey |
HRS-1893 |
Oral |
2 |
10.3 |
2,405 |
Predictable PK |
|
Monkey |
Mavacamten |
Oral |
2 |
78.3 |
3,521 |
Slow clearance, high accumulation |
Conclusion:
HRS-1893 (BHB-1893) has short, predictable half-life and minimal accumulation across species (rat, dog and monkey), supporting flexible BID dosing, whereas mavacamten shows long half-life and accumulation, requiring careful dose adjustments.
Phase I Clinical Data (ESC 2025)
HRS-1893 (BHB-1893) Phase I results were presented at the 2025 European Society of Cardiology (ESC) Congress. The trial evaluated safety, tolerability, pharmacokinetics (PK), pharmacodynamics (PD), and preliminary efficacy in patients with symptomatic oHCM.
Key findings:
- Population: Symptomatic oHCM patients with resting LVOT gradient ≥50 mmHg (or ≥30 mmHg with post-Valsalva ≥50 mmHg) and LVEF ≥60%.
- Design: Multiple ascending dose (MAD) study; doses: 60 mg and 80 mg orally twice daily for 14 days. Randomized 6:2 vs placebo.
- Safety: All adverse events were mild; no patients had LVEF <50%; mean LVEF changes <20% from baseline and returned to baseline after treatment.
- PK: Steady-state achieved by Day 8; accumulation ratios 1.30 (Cmax) and 2.04 (AUCtau).
- Efficacy / PD: Rapid and substantial reductions in LVOT gradient:
- Resting LVOT-G decreased 91% (71.2 → 6.0 mmHg) by Day 5
- Valsalva LVOT-G decreased 87.4% (66.6 → 8.0 mmHg) by Day 5
- All HRS-1893 patients had Valsalva LVOT-G ≤30 mmHg by Day 14
- N-terminal pro-B-type natriuretic peptide decreased ~60% from baseline
- Conclusion: HRS-1893 (BHB-1893) demonstrated favorable safety, PK, and hemodynamic efficacy, supporting 60 mg BID as the initial therapeutic dose and proof-of-concept in oHCM.
Phase III Clinical Development (NCT07021976)
BHB-1893 (formerly HRS-1893) has advanced into a Phase III trial in symptomatic obstructive HCM (oHCM) in China (NCT07021976), following earlier dose-ranging Phase II and clinical pharmacology studies, including a bridging study in Australia.
The Phase III study evaluates efficacy, safety, and long-term tolerability, with LVOT gradient reduction, NYHA functional improvement, and LVEF monitoring as primary and secondary endpoints.
Braveheart Bio plans to initiate global late-stage development in 2026, aiming to position BHB-1893 alongside aficamten (CK-274) and mavacamten (Camzyos) in the global myosin inhibitor landscape.
Deal Snapshot
| Item | Details |
|---|---|
| Asset | HRS-1893 (BHB-1893, selective cardiac myosin ATPase inhibitor) |
| Indication | Obstructive HCM |
| Licensor | Hengrui Pharma (China) |
| Licensee | Braveheart Bio (USA) |
| Upfront Payment | $65M ($32.5M cash + $32.5M equity) |
| Near-Term Milestone | $10M upon tech transfer completion |
| Long-Term Milestones | Up to $1.013B clinical + sales milestones |
| Royalties | On global sales outside Greater China |
| Governance | Joint Steering Committee (≤5 reps per party) |
| Effective | Upon execution; lasts through royalty period |
| Governing Law | New York State, USA |
Pipeline Comparison: HRS-1893 (BHB-1893) vs Aficamten vs Mavacamten
|
Feature |
HRS-1893 (BHB-1893) |
Aficamten (CK-274) |
Mavacamten (Camzyos) |
|
MOA |
Selective cardiac myosin ATPase inhibitor |
Selective cardiac myosin inhibitor |
Selective cardiac myosin inhibitor |
|
Target Indications |
Obstructive HCM |
Obstructive HCM; nHCM Phase III ongoing (ACACIA-HCM,
results pending) |
Obstructive HCM; nHCM Phase III failed |
|
Development & Regulatory Status |
Phase III ongoing (NCT07021976, oHCM) |
NDA submitted, PDUFA Dec 26, 2025 (US, oHCM) |
Approved US (2022), EU (2023) |
|
Administration |
Oral tablet |
Oral tablet |
Oral tablet |
|
Half-Life / PK |
Not publicly disclosed |
Shorter half-life, designed for titration |
Longer half-life, careful dosing required |
|
Developer / Sponsor |
Hengrui Pharma / Braveheart Bio (licensing) |
Cytokinetics |
Myokardia / BMS |
|
Strategic Model |
Out-licensing to NewCo (Braveheart Bio) |
Developed by Cytokinetics |
Developed by Myokardia / BMS |
Strategic Implications
- Braveheart Launch Context: The out-license coincided with Braveheart’s $185M Series A launch, supported by top-tier life science investors (Forbion, OrbiMed, RA Capital), establishing the company as a dedicated global platform for myosin-targeted cardiomyopathies.
- Global Expansion via NewCo: Hengrui leverages Braveheart Bio’s expertise and investor backing (Forbion, OrbiMed) to accelerate international development.
- Risk Sharing and Value Capture: Upfront payments, equity, milestones, and royalties balance near-term cash with long-term potential.
- Portfolio Synergy: HRS-1893 complements Hengrui’s expanding cardiovascular pipeline, including SHR-6934, SHR-4658, and HRS-9057, strengthening its presence in high-value cardiology innovation.
- Competitive Positioning: HRS-1893 is the first China-originated myosin inhibitor to pursue global licensing, differentiating it from aficamten and mavacamten, which are developed directly by their originating companies.
Market Context
Obstructive HCM is a genetic heart disease marked by left ventricular hypertrophy and hypercontractility, and it is a leading cause of sudden cardiac death in adolescents and athletes. Myosin inhibitors are now Class IB therapy in the 2025 Chinese HCM guidelines. If successfully developed and commercialized globally, HRS-1893 (BHB-1893) could become a major competitor in the expanding myosin inhibitor market.
Sources
- Jiangsu Hengrui Pharmaceuticals Co., Ltd. (2025). Entering into License Agreement for HRS-1893 with Braveheart Bio, Inc.
- ESC Congress 2025. Safety, tolerability, pharmacokinetics and pharmacodynamics of HRS-1893 in patients with obstructive hypertrophic cardiomyopathy: a randomized, double-blind, placebo-controlled phase 1 trial. Presented by L. Kang et al.
- Aficamten (CK-274) Discovery and Phase 3 Insights vs. Mavacamten in Hypertrophic Cardiomyopathy. Retrieved from SinoDrugWatch
- Chuang, C., et al. (2021). Discovery of Aficamten (CK-274), a Next-Generation Cardiac Myosin Inhibitor for the Treatment of Hypertrophic Cardiomyopathy. Journal of Medicinal Chemistry, 64(18), 13020–13035. doi:10.1021/acs.jmedchem.1c01290
- Garcia-Pavia, P., et al. (2025). Aficamten vs Metoprolol for Obstructive Hypertrophic Cardiomyopathy: Results from the MAPLE-HCM Trial. Journal of the American College of Cardiology, 75(6), 678–688. doi:10.1016/j.jacc.2024.11.011
- ClinicalTrials.gov. HRS-1893 in Obstructive Hypertrophic Cardiomyopathy (Phase III, NCT07021976).
- Patent: WO2022105852A1 – Triazine dione derivative, preparation method and application in medicine.
- Patent: CN117088854A – Pharmaceutically acceptable salts, crystal forms and preparation methods of triazinedione derivatives
Disclaimer: This article is for informational purposes only and does not constitute medical or investment advice. Readers should consult primary literature and official regulatory sources for verification.