HARMONi Phase 3: Ivonescimab Confirms PFS Benefit; OS Primary Endpoint Not Met, Nominal in Follow-Up Analysis
Summit Therapeutics’ Ivonescimab demonstrates statistically significant PFS improvement in the global HARMONi trial. OS did not meet the pre-specified threshold in the primary analysis, but longer follow-up in Western patients shows a nominal supportive trend for regulatory review.
At the 2025 World Conference on Lung Cancer (WCLC 2025), Summit Therapeutics reported updated results from the global Phase 3 HARMONi study (NCT06396065) evaluating ivonescimab (SMT112/AK112), a PD-1/VEGF bispecific antibody discovered by Akeso and co-developed with Summit. Ivonescimab, approved in China (Cardieva®) for NSCLC, showed a confirmed PFS benefit and updated OS results with longer follow-up in Western patients, as reported in Summit’s Sept 7, 2025 press release and the WCLC presentation.
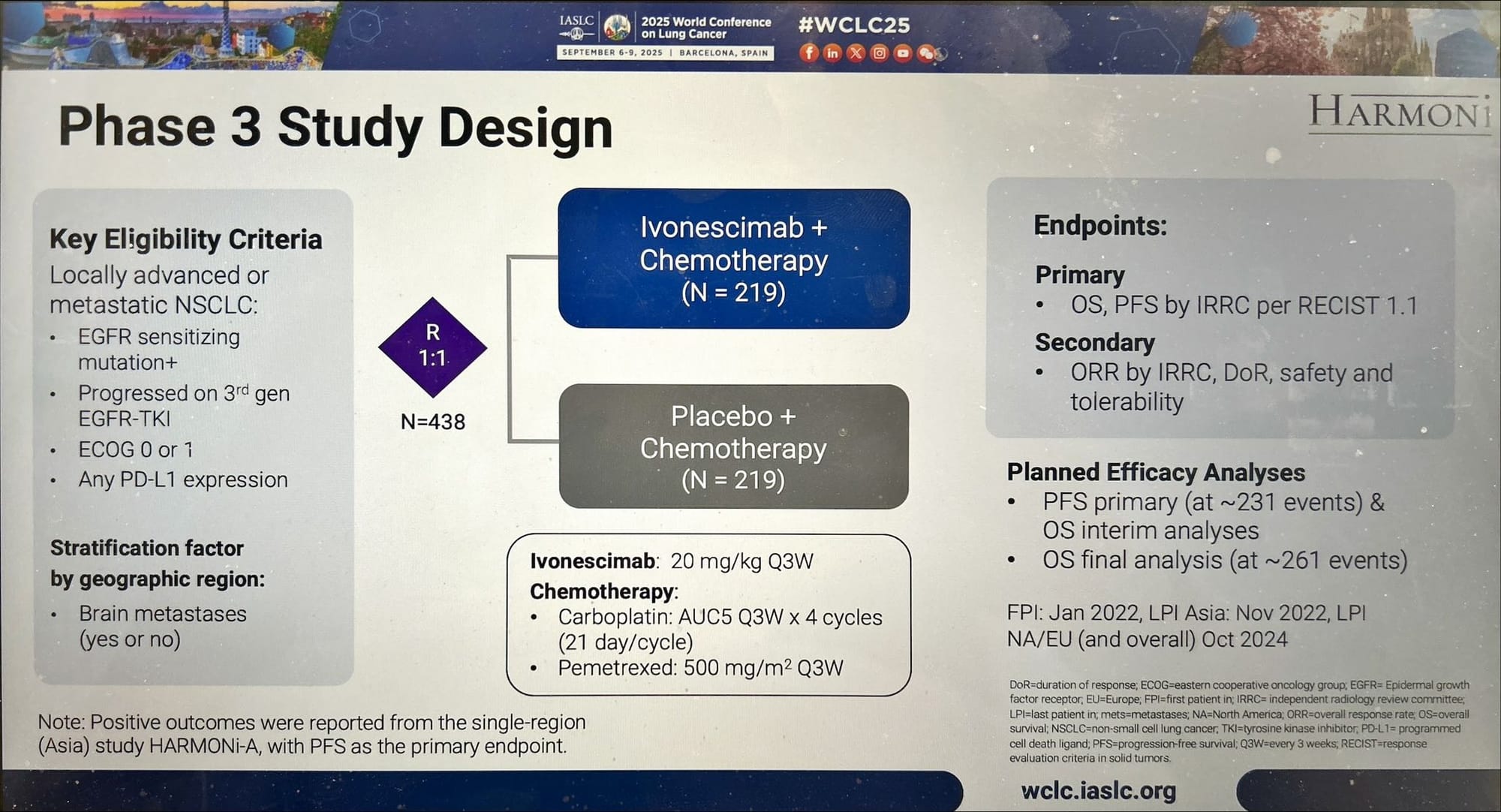
HARMONi Phase 3 Study Design
HARMONi tested ivonescimab plus chemotherapy versus chemotherapy alone in EGFR-mutated, locally advanced or metastatic non-squamous NSCLC after progression on osimertinib and platinum chemotherapy, a patient population with no globally accepted standard of care. The trial had dual primary endpoints: progression-free survival (PFS) and overall survival (OS).
HARMONi-A (China-Only) Context
HARMONi Phase 3: Ivonescimab + Chemotherapy – Efficacy & Safety (Global & Regional)
|
Endpoint |
Ivonescimab + Chemo |
Placebo + Chemo |
HR (95% CI) |
p-value / Notes |
|
Median PFS (months, ITT) |
6.8 mos (n=219) |
4.4 mos (n=219) |
0.52 (0.41–0.66) |
p < 0.00001; BICR-assessed |
|
ORR |
45% |
34% |
– |
– |
|
Median DoR (months) |
7.6 mos |
4.2 mos |
– |
– |
|
OS Primary Analysis (Apr 2025, ITT) |
16.8 mos (n=219) |
14.0 mos (n=219) |
0.79 (0.62–1.01) |
p = 0.057; did not meet SAP threshold (p ≤
0.0448) |
|
OS Follow-Up Analysis (Sept 2025, ITT) |
16.8 mos (n=219) |
14.0 mos (n=219) |
0.78 (0.62–0.98) |
Nominal p = 0.0332; exploratory/supportive |
|
OS by Region (Follow-Up, ITT) |
||||
|
North America |
Not reached (n=43) at DCO* |
14.0 mos (n=50) |
0.70 |
– |
|
Europe |
17.0 mos (n=39) |
14.0 mos (n=41) |
Improved vs primary |
– |
|
Asia |
16.7 mos (n=136) |
14.0 mos (n=137) |
0.76 |
– |
|
TRAEs Grade 3+ |
50.0% |
42.2% |
– |
Most frequent: anemia and decreases in white blood cell
count, neutrophil count, and platelet count |
|
TRAEs Leading to Discontinuation |
7.3% |
5.0% |
– |
– |
|
TRAEs Leading to Death |
1.8% |
2.3% |
– |
– |
|
Grade 3+ Immune-related AEs |
9.6% |
2.3% |
– |
– |
|
Grade 3+ VEGF-related AEs |
7.3% |
6.0% |
– |
– |
*North American patients are a subset of Western patients. ITT = intention-to-treat; BICR = blinded independent central review; TRAEs = treatment-related adverse events.
Note: Nominal p-value is from an exploratory follow-up analysis and is not pre-specified in the statistical analysis plan (SAP). The SAP threshold represents the pre-defined significance level required to formally declare statistical significance (OS SAP threshold: p ≤ 0.0448). Nominal p-values indicate supportive trends but are not confirmatory.
HARMONi-A (China-Only) Context
- Population: Exclusively Chinese patients with EGFR-mutated NSCLC post-third-generation EGFR TKI therapy.
- Results: Final OS analysis showed a statistically significant OS benefit, reinforcing the PFS benefit observed in both HARMONi-A and global HARMONi trials.
- Interpretation: While HARMONi-A provides supportive evidence of ivonescimab efficacy in Asian patients, differences in tumor biology, prior therapies, and genetics may limit direct extrapolation to Western populations.
Discussion
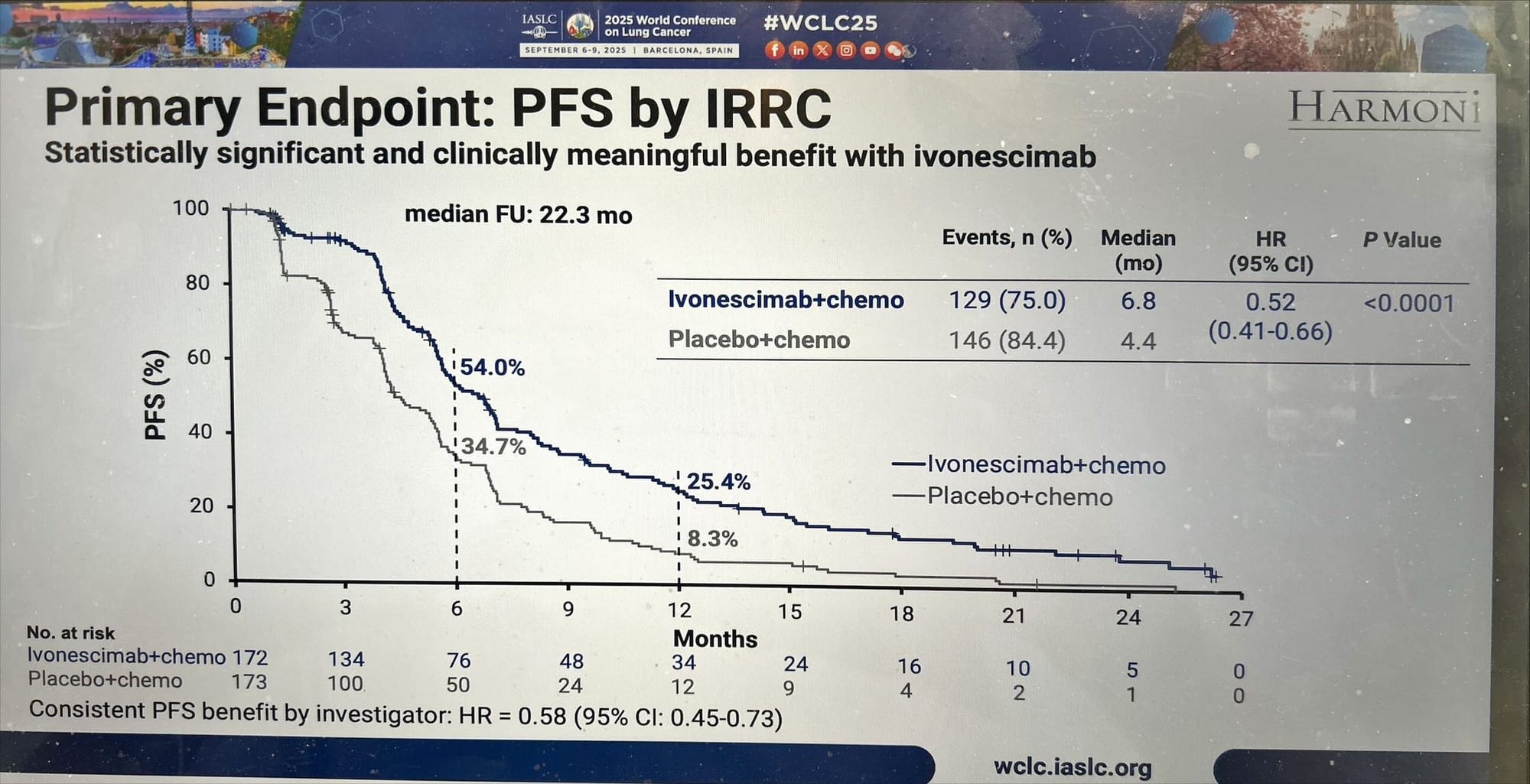
1. Progression-Free Survival (Primary Endpoint, Met)
- Median PFS: 6.8 months (ivonescimab + chemo) vs 4.4 months (placebo + chemo)
- Hazard ratio (HR): 0.52, p < 0.00001
- Benefit consistent across Asian and Western subgroups, showing a clear, highly significant improvement.
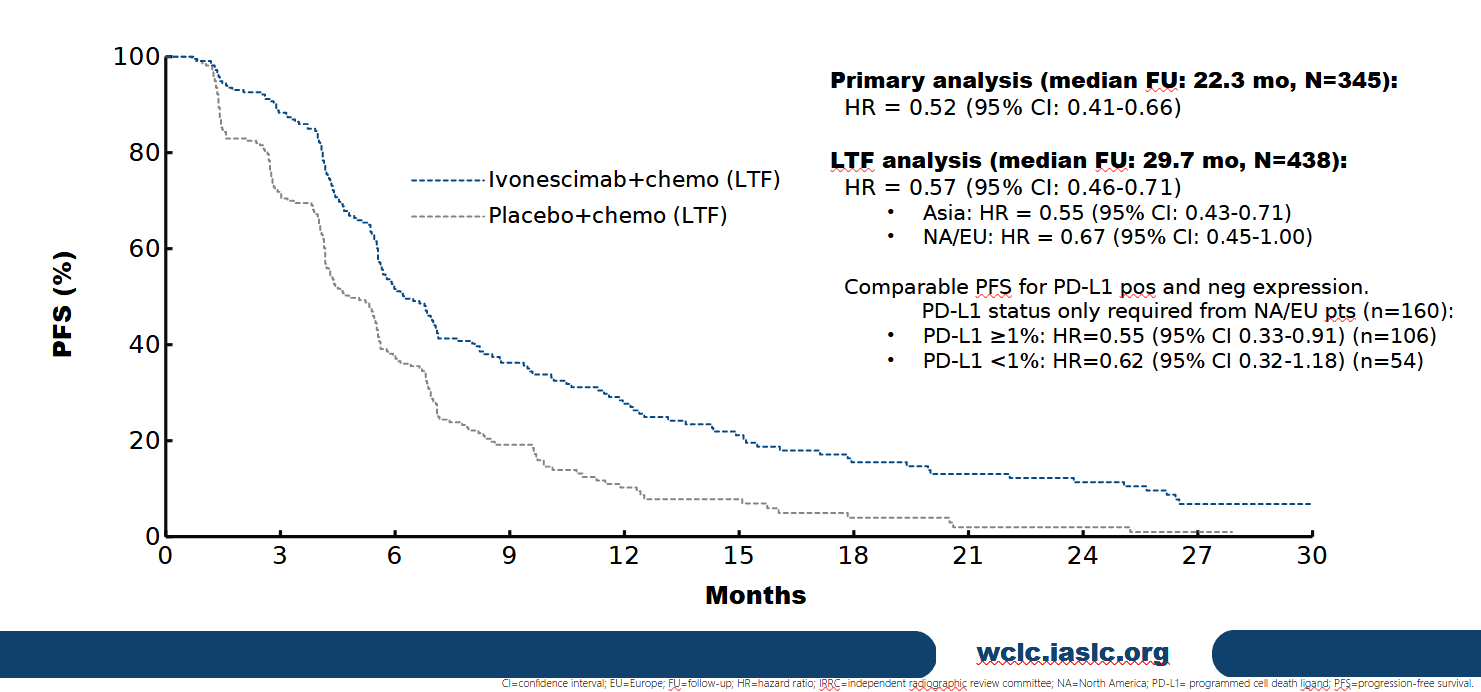
2. Overall Survival (OS)
- Primary OS analysis (Apr 2025, ITT): HR 0.79, p = 0.057 — did not meet the pre-specified SAP threshold (p ≤ 0.0448).
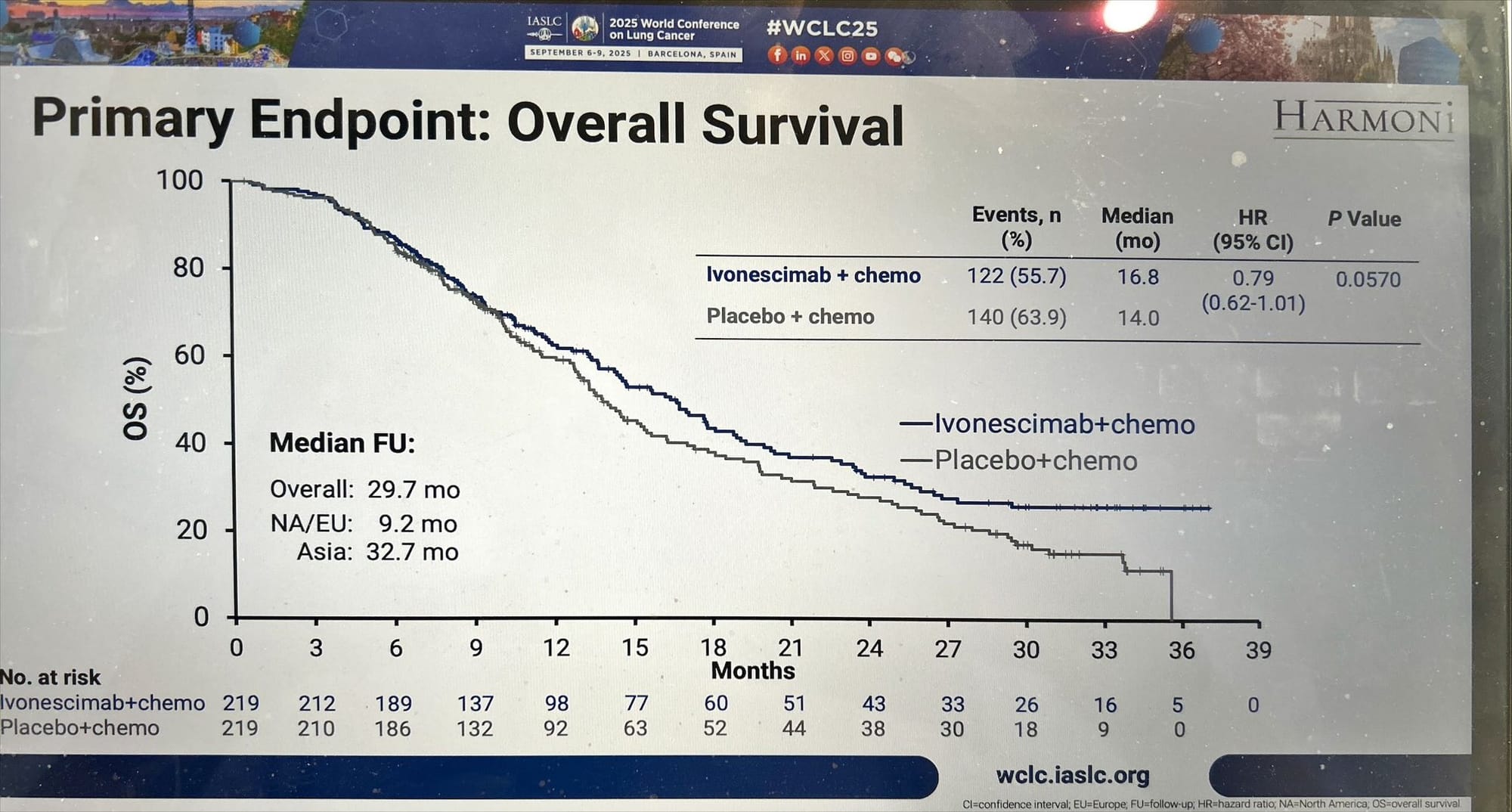
- Follow-up analysis (Sept 2025, longer Western follow-up): HR 0.78, nominal p = 0.0332 — exploratory/supportive; median OS unchanged from primary analysis. The nominal p-value observed in Western patients is exploratory and not pre-specified in the statistical analysis plan, and therefore is considered a supportive trend but lacks statistical confirmation.
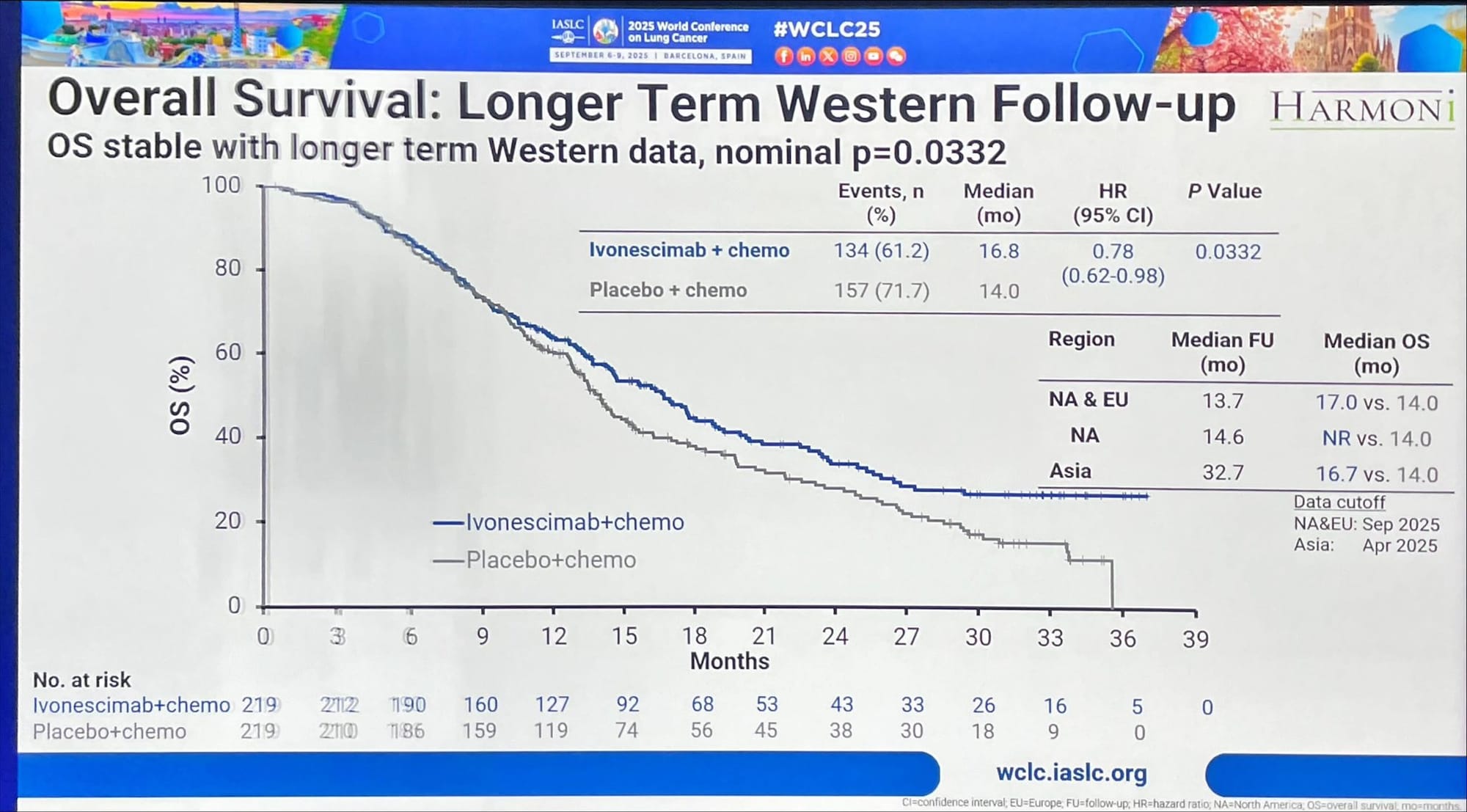
- Regional trends: Western and North American patients showed improved HRs vs primary analysis, while Asian patients had consistent OS (16.7 mos vs 14.0 mos).
3. Regulatory Perspective
· Nominal OS trend vs PFS benefit: Western regulators (FDA/EMA) would likely consider the nominal OS trend as supportive evidence rather than confirmatory, because it does not meet the pre-specified SAP threshold. In contrast, the PFS benefit is statistically confirmed, strengthening the overall efficacy profile of ivonescimab.
· Implications for labeling: PFS data may support efficacy claims, but regulators may require additional OS confirmation before granting full OS labeling in Western markets. Ongoing studies, including HARMONi-3 and HARMONi-7 (first-line NSCLC), will provide further efficacy and OS data, although in a different patient population than the post-osimertinib setting.
· US regulatory strategy: Summit holds Fast Track designation. A US filing is possible, but the FDA may request longer follow-up or pooled analyses from HARMONi or other relevant trials to substantiate OS benefit.
· EMA considerations: The European Medicines Agency would likely take a similar approach, emphasizing PFS as the primary endpoint, while considering OS exploratory. Differences in patient populations and regional data maturity (Western vs Asian) may influence approval strategy and labeling claims.
4. Ongoing Trials & Key Considerations
- HARMONi-3: Ivonescimab + chemo vs pembrolizumab + chemo in first-line metastatic NSCLC.
- HARMONi-7: Ivonescimab monotherapy vs pembrolizumab in first-line NSCLC with high PD-L1 expression.
- HARMONi-2, HARMONi-6: Phase III single-region trials in China, providing further safety and efficacy data.
Key considerations:
- OS trends in Western patients are still maturing; HARMONi-A shows robust OS in Chinese patients.
- Consistency of PFS benefit across trials reinforces clinical activity.
- Regulatory strategy: Combining global HARMONi results with supportive HARMONi-A data may strengthen submissions, but OS labeling claims in Western markets will likely rely on confirmatory multinational data.
Regulatory & Competitive Landscape
- China: Ivonescimab approved as Cardieva®, giving Akeso a first-mover advantage.
- Global: HARMONi is the first pivotal PD-1/VEGF readout in post-osimertinib, post-platinum EGFRm NSCLC.
- Competitors: Regeneron’s odronextamab and Innovent/Sino’s IBI318 are earlier in global development. Summit is leveraging its head start and China approval to establish a global franchise.
Conclusion
- Confirmed PFS benefit demonstrates meaningful clinical activity in EGFRm NSCLC.
- OS primary endpoint formally not met; follow-up shows a nominal supportive trend, particularly in Western and North American patients.
- HARMONi-A supports efficacy in Chinese patients, highlighting potential regional differences.
- Ongoing global trials will provide additional data to inform regulatory submissions in multiple regions.
Sources
· Goldman, J. W.; et al. Ivonescimab vs. Placebo Plus Chemotherapy in EGFR-Mutated NSCLC Progressed After Third-Generation EGFR-TKI Treatment: HARMONi. J. Thorac. Oncol. 2025, 20 (9), 1445–1455. DOI: 10.1016/j.jtho.2025.06.012.
· Xiong, A.; et al. Ivonescimab vs. Pembrolizumab in PD-L1-Positive Advanced NSCLC: A Phase 3 Randomized Trial. Lancet Oncol. 2025, 26 (4), 321–329. DOI: 10.1016/S1470-2045(25)00045-6.
· Summit Therapeutics. Ivonescimab Data from Global Phase III HARMONi Study to be Showcased at Presidential Symposium at WCLC 2025. Summit Therapeutics Press Release, August 14, 2025.
· Summit Therapeutics. Ivonescimab Plus Chemotherapy Demonstrates Consistent Global Benefit: HARMONi Data Update Shows OS HR 0.78, Nominal p = 0.0332. Summit Therapeutics Press Release, September 7, 2025.
· International Association for the Study of Lung Cancer. Press Release. Ivonescimab Plus Chemotherapy Improves Progression-Free Survival in Patients with EGFR+ NSCLC Following 3rd-Generation EGFR-TKI Therapy. September 7, 2025.
· ClinicalTrials.gov: NCT06396065
Disclaimer: This article is for informational purposes only and does not constitute medical or investment advice. Readers should consult primary literature and official regulatory sources for verification.