FDA Novel Drug Approvals for 2025: Precision Therapies, Rare Diseases, and China’s Growing Footprint
As of September 30, 2025, the FDA has approved 32 novel drugs, emphasizing precision medicine, rare disease advances, and a notable increase in China-origin therapies gaining U.S. approval. This analysis covers key drugs, modalities, and the evolving China biotech footprint.
Updated September 30, 2025, added Rhapsido (remibrutinib), September 25, 2025, added PALSONIFY (paltusotine), Inluriyo (imlunestrant) and Keytruda Qlex (pembrolizumab and berahyaluronidase alfa-pmph); September 20, 2025, added Forzinity (elamipretide); August 29, 2025, added Wayrilz (rilzabrutinib); August 22, 2025, added DAWNZERA
The U.S. FDA has approved 32 novel drugs in 2025 to date, spanning oncology, infectious diseases, neurology, hematology, nephrology, ophthalmology, and pain management. This year’s approvals underscore three key themes:
- Expanding precision medicine in oncology
- First-in-class mechanisms for non-opioid pain control
- Breakthroughs in rare disease therapeutics
The approvals, detailed on the FDA’s Novel Drug Approvals 2025 page, also highlight a growing presence of China-origin assets securing global positions — most notably Dizal Pharmaceutical’s sunvozertinib for EGFR exon20 insertion NSCLC.
Modality Distribution
FDA Novel Drug Approvals, Jan 1 – Sep 30, 2025
- Small Molecules – 21
- Monoclonal Antibodies – 4
- Antibody-Drug Conjugates (ADCs) – 2
- siRNA – 1
- Bispecific Antibody – 1
- Combination Therapy – 2
- Oligonucleotide/ASO – 1
Small molecules dominating, alongside a steady rise in biologics and novel modalities.
Key Achievements in 2025
- Precision Oncology — Seven approvals target defined genetic subtypes:
- ROS1+ NSCLC (taletrectinib)
- KRAS-mutant ovarian cancer (avutometinib/defactinib)
- c-Met overexpressing NSCLC (telisotuzumab vedotin)
- EGFR exon20ins NSCLC (sunvozertinib, China-origin)
- HER2-negative breast cancer (datopotamab deruxtecan)
- HER2-mutant NSCLC (zongertinib)
- ER-positive, HER2-negative, ESR1-mutated breast cancer (imlunestrant)
- Non-Opioid Analgesic Breakthrough — Vertex’s suzetrigine (Journavx), the first NaV1.8 inhibitor approved for acute pain, marks a novel approach in pain management; however, label expansion to chronic pain faces FDA scrutiny.
- Rare Disease Advancements —
- Mirdametinib for adult neurofibromatosis type 1 plexiform neurofibromas
- Fitusiran (siRNA) for hemophilia A/B
- Garadacimab for hereditary angioedema prevention
- Modeyso (dordaviprone) for H3 K27M–mutant diffuse midline glioma
- BRINSUPRI (brensocatib) for non-cystic fibrosis bronchiectasis, the first approved therapy for this chronic lung disease
- DAWNZERA (donidalorsen) for hereditary angioedema prophylaxis
- Wayrilz (rilzabrutinib) – First-in-class BTK inhibitor for adult ITP, addressing systemic immune dysregulation and improving quality of life beyond platelet count normalization
- Forzinity (elamipretide) for Barth syndrome, the first mitochondrial-targeted therapy for this ultra-rare genetic disorder causing severe heart failure and fatigue in males
- PALSONIFY (paltusotine) for acromegaly, the first oral, once-daily SST2 agonist for this rare pituitary disorder
- Combatting Antimicrobial Resistance — Gepotidacin introduces a first-in-class oral antibiotic targeting resistant uropathogens.
- Pediatric and Preventive Focus — RSV prevention (clesrovimab) and siRNA-based prophylaxis (fitusiran) expand options for infants and children.
- Autoimmune & Allergy Innovation —Rhapsido (remibrutinib), the first oral BTK inhibitor for chronic spontaneous urticaria (CSU), expands targeted options for allergic di seases and represents a major milestone in immunology.
Notable 2025 Approvals
- Journavx (suzetrigine) — First-in-class NaV1.8 blocker, redefining acute pain management.
- Gomekli (mirdametinib) — First NF1-PN therapy with strong overall response rates in adults and pediatrics.
- Blujepa (gepotidacin) — Novel antibiotic active against resistant urinary tract infections.
- Qfitlia (fitusiran) — First siRNA therapy for hemophilia, enabling simplified prophylaxis.
- Imaavy (nipocalimab) — Anti-FcRn monoclonal antibody improving myasthenia gravis symptoms.
- Ibtrozi (taletrectinib) — CNS-penetrant ROS1/TRK inhibitor for NSCLC.
- Enflonsia (clesrovimab) — Single-dose RSV prophylaxis reducing infant hospitalizations.
- Zegfrovy (sunvozertinib) — China’s Dizal Pharmaceutical wins first U.S. approval for an EGFR exon20 insertion oral targeted therapy.
- Modeyso (Dordaviprone) - First FDA approval of a systemic therapy for H3 K27M-mutant diffuse midline glioma (DMG, formerly DIPG), offering new hope in this devastating pediatric brain tumor.
- Hernexeos (zongertinib) — First oral targeted therapy for HER2-mutant NSCLC, showing 75% ORR in phase 1 with durable responses and improved tolerability over EGFR-targeting TKIs.
- BRINSUPRI (brensocatib) — First and only DPP1 inhibitor approved for non-cystic fibrosis bronchiectasis, reducing exacerbations by 21% (10 mg) and 20% (25 mg) in the phase 3 ASPEN trial, addressing neutrophilic inflammation.
- DAWNZERA (donidalorsen) — First RNA-targeted prophylactic for hereditary angioedema, with Q4W or Q8W dosing and 81-87% attack reduction in Phase 3 OASIS-HAE trial.
- Wayrilz (rilzabrutinib) – Oral BTK inhibitor for ITP; FDA-approved Aug 29, 2025; shows rapid/durable platelet response and symptom relief in patients refractory to prior therapy.
- Forzinity (elamipretide) — First-in-class mitochondrial stabilizer for Barth syndrome, improving muscle strength and function in this life-limiting pediatric-onset disease; accelerated approval based on knee extensor improvements, with post-approval confirmatory trials required.
- Keytruda Qlex (pembrolizumab and berahyaluronidase alfa-pmph):First subcutaneous formulation of pembrolizumab, reducing administration time to 1–2 minutes compared to 30 minutes for IV Keytruda. Approved for 38 solid tumor indications, with comparable efficacy and safety (MK-3475A-D77 trial), enhancing patient convenience and healthcare efficiency.
- PALSONIFY (paltusotine) — First once-daily oral SST2 agonist for acromegaly, demonstrating rapid biochemical control and symptom reduction in PATHFNDR-1/2 trials, offering a convenient alternative to injectable therapies.
- Rhapsido (remibrutinib) — Novartis’ oral BTK inhibitor, the first targeted therapy approved for chronic spontaneous urticaria (CSU). Accelerated approval highlights its role as a novel immunology therapy; the China NDA was accepted with priority review in March 2025.
China-Linked Highlights
- Zegfrovy (sunvozertinib) — First U.S. approval for Dizal Pharmaceutical; marks a milestone for Chinese biotech innovation in lung cancer precision therapy.
- Penpulimab — Akeso’s PD-1 antibody gained U.S. approval in nasopharyngeal carcinoma, joining about 10 PD-1/PD-L1 inhibitors already on the market. The FDA nod positions Penpulimab in direct competition with Coherus BioSciences and Junshi Biosciences’ China-origin PD-1 Loqtorzi, the first FDA-approved PD-1 for nasopharyngeal carcinoma in 2023, highlighting China’s growing footprint in immuno-oncology.
- Taletrectinib — Developed by China’s AnHeart Therapeutics in collaboration with Innovent Biologics, originally discovered by Daiichi Sankyo, and now with Novation holding global commercial rights, this CNS-penetrant ROS1/TRK inhibitor secured FDA approval for ROS1+ NSCLC. It illustrates the increasingly complex and global nature of drug development involving Chinese biotech players.
Full 2025 Approval List (Jan 1 – Sep 25)
A comprehensive table including drug name, active ingredient, approval date, indication, modality, mechanism of action, IUPAC name, SMILES, sponsor, and clinical significance (where applicable) is available here (28 entries). The list will be updated with new approvals before year-end.
- Drug Name (Brand Name): Datroway
Generic Name: datopotamab deruxtecan-dlnk
Structure:
Approval Date: 1/17/2025
Indication: HER2-negative, HR-positive metastatic breast cancer
Modality: ADC (antibody-drug conjugate)
MOA/Target: TROP2-directed ADC
IUPAC Name: N/A (biologic)
SMILES: N/A (biologic)
Sponsor: Daiichi Sankyo/AstraZeneca - Drug Name (Brand Name): Grafapex
Generic Name: treosulfan
Structure:
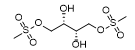
Approval Date: 1/21/2025
Indication: Stem cell transplant preparative regimen
Modality: Small molecule
MOA/Target: Alkylating agent
IUPAC Name: [(2S,3S)-2,3-dihydroxy-4-methylsulfonyloxybutyl] methanesulfonate
SMILES: COCS(=O)(=O)OCC(O)C(O)C(O)COS(=O)(=O)OC
Sponsor: Medac
- Drug Name (Brand Name): Journavx
Generic Name: suzetrigine
Structure:
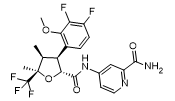
Approval Date: 1/30/2025
Indication: Moderate-severe acute pain
Modality: Small molecule
MOA/Target: Voltage-gated sodium channel blocker
IUPAC Name: 4-[[(2R,3S,4S,5R)-3-(3,4-difluoro-2-methoxyphenyl)-4,5-dimethyl-5-(trifluoromethyl)oxolane-2-carbonyl]amino]pyridine-2-carboxamide
SMILES:C[C@H]1[C@H]([C@@H](O[C@@]1(C)C(F)(F)F)C(=O)NC2=CC(=NC=C2)C(=O)N)C3=C(C(=C(C=C3)F)F)OC
Sponsor: Vertex
Significance: First-in-class non-opioid analgesic for moderate-to-severe acute pain, addressing the opioid crisis via NaV1.8 inhibition. Efforts to expand its label to chronic pain face FDA hurdles, and a follow-on candidate, VX-993, was discontinued after failing a phase 2 acute pain trial.
- Drug Name (Brand Name): Gomekli
Generic Name: mirdametinib
Structure:
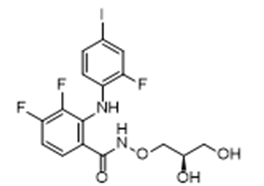
Approval Date: 2/11/2025
Indication: NF1 with plexiform neurofibromas
Modality: Small molecule
MOA/Target: MEK1/2 inhibitor
IUPAC Name: N-[(2R)-2,3-dihydroxypropoxy]-3,4-difluoro-2-(2-fluoro-4-iodoanilino)benzamide
SMILES: C1=CC(=C(C=C1I)F)NC2=C(C=CC(=C2F)F)C(=O)NOC[C@@H](CO)O
Sponsor: SpringWorks
Significance: First approved therapy for adult NF1-PN, with 41% ORR in adults and 52% in pediatrics (ReNeu trial), addressing a rare condition with significant morbidity.
- Drug Name (Brand Name): Romvimza
Generic Name: vimseltinib
Structure:
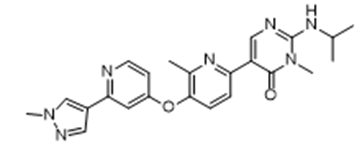
Approval Date: 2/14/2025
Indication: Symptomatic tenosynovial giant cell tumor
Modality: Small molecule
MOA/Target: CSF1R inhibitor
IUPAC Name: 3-methyl-5-[6-methyl-5-[2-(1-methylpyrazol-4-yl)pyridin-4-yl]oxypyridin-2-yl]-2-(propan-2-ylamino)pyrimidin-4-one
SMILES: CC1=C(C=CC(=N1)C2=CN=C(N(C2=O)C)NC(C)C)OC3=CC(=NC=C3)C4=CN(N=C4)C
Sponsor: Deciphera
- Drug Name (Brand Name): Blujepa
Generic Name: gepotidacin
Structure:
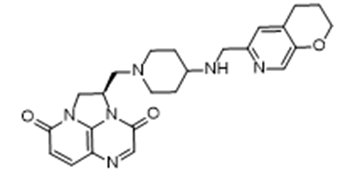
Approval Date: 3/25/2025
Indication: Uncomplicated UTI
Modality: Small molecule
MOA/Target: Bacterial topoisomerase II inhibitor
IUPAC Name: (2R)-2-({4-[(3,4-Dihydro-2H-pyrano[2,3-c]pyridin-6-ylmethyl)amino]-1-piperidinyl}methyl)-1,2-dihydro-3H,8H-2a,5,8a-triazaacenaphthylene-3,8-dione
SMILES: C1CC2=CC(=NC=C2OC1)CNC3CCN(CC3)C[C@@H]4CN5C(=O)C=CC6=C5N4C(=O)C=N6
Sponsor: GSK
Significance: First-in-class antibiotic, effective against resistant uropathogens (EAGLE-2/3 trials), addressing antimicrobial resistance.
- Drug Name (Brand Name): Qfitlia
Generic Name: fitusiran
Structure:
Approval Date: 3/28/2025
Indication: Hemophilia A or B
Modality: siRNA
MOA/Target: siRNA targeting antithrombin
IUPAC Name: N/A (oligonucleotide)
SMILES: N/A (oligonucleotide)
Sponsor: Sanofi
Significance: First siRNA for hemophilia, reducing annualized bleeding rate by 71-73% in phase 3 trials, simplifying prophylaxis with bimonthly dosing.
- Drug Name (Brand Name): Vanrafia
Generic Name: atrasentan
Structure:
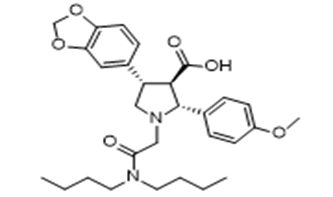
Approval Date: 4/2/2025
Indication: IgA nephropathy
Modality: Small molecule
MOA/Target: Endothelin A receptor antagonist
IUPAC Name: (2R,3R,4S)-4-(1,3-benzodioxol-5-yl)-1-[2-(dibutylamino)-2-oxoethyl]-2-(4-methoxyphenyl)pyrrolidine-3-carboxylic acid
SMILES:CCCCN(CCCC)C(=O)CN1C[C@@H]([C@H]([C@@H]1C2=CC=C(C=C2)OC)C(=O)O)C3=CC4=C(C=C3)OCO4
Sponsor: Chinook/Roche
- Drug Name (Brand Name): penpulimab-kcqx
Generic Name: penpulimab-kcqx
Structure:
Approval Date: 4/23/2025
Indication: Nasopharyngeal carcinoma
Modality: Monoclonal antibody
MOA/Target: Anti-PD-1 antibody
IUPAC Name: N/A (biologic)
SMILES: N/A (biologic)
Sponsor: Akeso - Drug Name (Brand Name): Imaavy
Generic Name: nipocalimab-aahu
Structure:
Approval Date: 4/29/2025
Indication: Generalized myasthenia gravis
Modality: Monoclonal antibody
MOA/Target: Anti-FcRn antibody
IUPAC Name: N/A (biologic)
SMILES: N/A (biologic)
Sponsor: J&J
Significance: Improves MG-ADL score by 4.70 points (VIVACITY-MG3 trial), enhancing muscle function and quality of life for myasthenia gravis patients.
- Drug Name (Brand Name): Avmapki Fakzynja Co-Pack
Generic Name: avutometinib / defactinib
Structure:
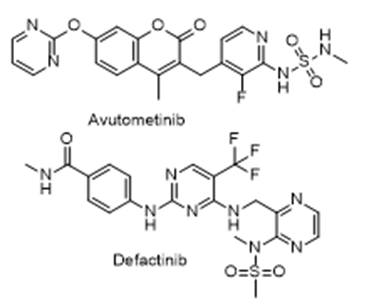
Approval Date: 5/8/2025
Indication: KRAS-mutant ovarian cancer
Modality: Combination (small molecules)
MOA/Target: MEK inhibitor + FAK inhibitor
IUPAC Name:
- Avutometinib: N-(1-methyl-5-(2-(5-(trifluoromethyl)-1H-imidazol-2-yl)pyridin-4-yloxy)pyridin-2-yl)-2-(3-(trifluoromethyl)phenyl)acetamide
- Defactinib: N-methyl-4-({4-[({3-[methyl(methylsulfonyl)amino]pyridin-2-yl}methyl)amino]-5-(trifluoromethyl)pyrimidin-2-yl}amino)benzamide
SMILES:
- Avutometinib: Cc1c(c(c(c(n1)NC(=O)Cc2cc(cc(c2)C(F)(F)F)C(F)(F)F)Oc3ccnc(c3)c4[nH]c(c(n4)C(F)(F)F)C)C
- Defactinib: CNC(=O)c1ccc(cc1)Nc2ncc(c(n2)NCc3c(c(c(cn3)N(C)S(=O)(=O)C)C(F)(F)F)C
Sponsor: Verastem
- Drug Name (Brand Name): Emrelis
Generic Name: telisotuzumab vedotin-tllv
Structure:
Approval Date: 5/14/2025
Indication: NSCLC with c-Met overexpression
Modality: ADC (antibody-drug conjugate)
MOA/Target: c-Met-directed ADC
IUPAC Name: N/A (ADC/biologic)
SMILES: N/A (ADC/biologic)
Sponsor: AbbVie/Seagen - Drug Name (Brand Name): Tryptyr
Generic Name: acoltremon
Structure:
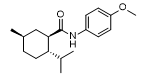
Approval Date: 5/28/2025
Indication: Dry eye disease
Modality: Small molecule
MOA/Target: Neurotrophic factor receptor modulator
IUPAC Name: (1R,2S,5R)-N-(4-methoxyphenyl)-5-methyl-2-propan-2-ylcyclohexane-1-carboxamide
SMILES: C[C@@H]1CC[C@H]([C@@H](C1)C(=O)NC2=CC=C(C=C2)OC)C(C)C
Sponsor: Novaliq
- Drug Name (Brand Name): Enflonsia
Generic Name: clesrovimab-cfor
Structure:
Approval Date: 6/9/2025
Indication: RSV prevention in infants
Modality: Monoclonal antibody
MOA/Target: Anti-RSV antibody
IUPAC Name: N/A (biologic)
SMILES: N/A (biologic)
Sponsor: Janssen
Significance: Reduces RSV-associated hospitalizations by 84.3% (CLEVER trial), offering single-dose prophylaxis for infants
- Drug Name (Brand Name): Ibtrozi
Generic Name: taletrectinib
Structure:
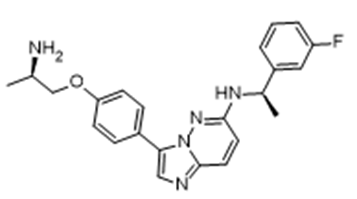
Approval Date: 6/11/2025
Indication: ROS1+ NSCLC
Modality: Small molecule
MOA/Target: ROS1 inhibitor
IUPAC Name: N-(5-((4-(1H-indol-3-yl)pyrimidin-2-yl)amino)-2-fluorophenyl)-4-(1-methyl-1H-pyrazol-4-yl)thiazol-2-amine
SMILES: Cn1cc(c(n1)C)Sc2ncc(s2)Nc3cc(c(c(c3)F)Nc4nccc(n4)c5c[nH]c6c5cccc6)
Sponsor: AnHeart
Significance: Next-generation ROS1 inhibitor with high CNS penetration, achieving 71% ORR and 14.1-month DOR (TRUST-I/II trials) for NSCLC.
- Drug Name (Brand Name): Andembry
Generic Name: garadacimab-gxii
Structure:
Approval Date: 6/16/2025
Indication: Prevent hereditary angioedema attacks
Modality: Monoclonal antibody
MOA/Target: Anti-FXII monoclonal antibody
IUPAC Name: N/A (biologic)
SMILES: N/A (biologic)
Sponsor: CSL Behring - Drug Name (Brand Name): Lynozyfic
Generic Name: linvoseltamab-gcpt
Structure:
Approval Date: 7/2/2025
Indication: Multiple myeloma (after ≥4 prior therapies)
Modality: Bispecific antibody
MOA/Target: CD38-targeted bispecific antibody
IUPAC Name: N/A (biologic)
SMILES: N/A (biologic)
Sponsor: Regeneron - Drug Name (Brand Name): Zegfrovy
Generic Name: sunvozertinib
Structure:
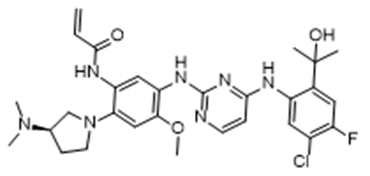
Approval Date: 7/2/2025
Indication: NSCLC, EGFR exon 20 mutation
Modality: Small molecule
MOA/Target: EGFR exon 20 inhibitor
IUPAC Name: N-[5-[[4-[5-chloro-4-fluoro-2-(2-hydroxypropan-2-yl)anilino]pyrimidin-2-yl]amino]-2-[(3R)-3-(dimethylamino)pyrrolidin-1-yl]-4-methoxyphenyl]prop-2-enamide
SMILES: CC(C)(C1=CC(=C(C=C1NC2=NC(=NC=C2)NC3=C(C=C(C(=C3)NC(=O)C=C)N4CCC@HN(C)C)OC)Cl)F)O
Sponsor: Dizal Pharmaceutical
Significance: First and only FDA-approved oral targeted therapy for EGFR exon20ins NSCLC, developed by China’s Dizal Pharmaceutical.
- Drug Name (Brand Name): Ekterly
Generic Name: sebetralstat
Structure:
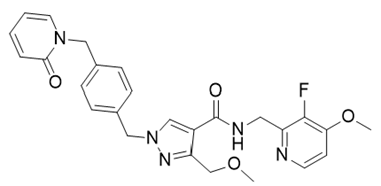
Approval Date: 7/3/2025
Indication: Acute hereditary angioedema attacks
Modality: Small molecule
MOA/Target: Plasma kallikrein inhibitor
IUPAC Name: N-[(3-fluoro-4-methoxypyridin-2-yl)methyl]-3-(methoxymethyl)-1-[[4-[(2-oxopyridin-1-yl)methyl]phenyl]methyl]pyrazole-4-carboxamide
SMILES: COCC1=NN(C=C1C(=O)NCC2=NC=CC(=C2F)OC)CC3=CC=C(C=C3)CN4C=CC=CC4=O
Sponsor: KalVista
- Drug Name (Brand Name): Anzupgo
Generic Name: delgocitinib
Structure:
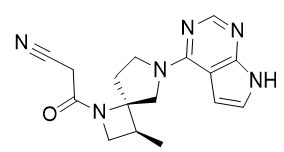
Approval Date: 7/23/2025
Indication: Chronic hand eczema
Modality: Small molecule
MOA/Target: Pan-JAK inhibitor
IUPAC Name: 3-[(3S,4R)-3-methyl-7-(7H-pyrrolo[2,3-d]pyrimidin-4-yl)-1,7-diazaspiro[3.4]octan-1-yl]-3-oxopropanenitrile
SMILES: C[C@H]1CN([C@]12CCN(C2)C3=NC=NC4=C3C=CN4)C(=O)CC#N
Sponsor: Japan Tobacco
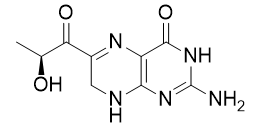
- Drug Name (Brand Name): Sephience
Generic Name: sepiapterin
Structure:
Approval Date: 7/28/2025
Indication: Hyperphenylalaninemia in PKU
Modality: Small molecule
MOA/Target: Tetrahydrobiopterin precursor
IUPAC Name: 2-amino-6-[(2S)-2-hydroxypropanoyl]-7,8-dihydro-3H-pteridin-4-one
SMILES: C[C@@H](C(=O)C1=NC2=C(NC1)N=C(NC2=O)N)O
Sponsor: PTC Therapeutics
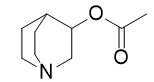
- Drug Name (Brand Name): Vizz
Generic Name: aceclidine
Structure:
Approval Date: 7/31/2025
Indication: Presbyopia
Modality: Small molecule
MOA/Target: Muscarinic receptor agonist
IUPAC Name: 1-azabicyclo[2.2.2]octan-3-yl acetate
SMILES: CC(=O)OC1CN2CCC1CC2
Sponsor: Visus Therapeutics
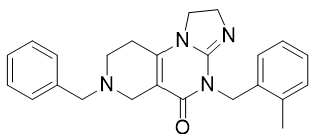
- Drug Name (Brand Name): Modeyso
Generic Name: dordaviprone
Structure:
Approval Date: 8/6/2025
Indication: H3 K27M–mutant diffuse midline glioma
Modality: Small molecule
MOA/Target: Antagonist of dopamine receptor D2/3 (DRD2/3) and agonist of mitochondrial caseinolytic protease P (ClpP).
IUPAC Name: 11-benzyl-7-[(2-methylphenyl)methyl]-2,5,7,11-tetrazatricyclo[7.4.0.02,6]trideca-1(9),5-dien-8-one
SMILES: CC1=CC=CC=C1CN2C(=O)C3=C(CCN(C3)CC4=CC=CC=C4)N5C2=NCC5
Sponsor: Jazz Pharmaceuticals (acquired Chimerix in April 2025)
Significance: First systemic therapy FDA-approved for H3 K27M-mutant DMG, a previously untreatable aggressive midline brain tumor predominantly affecting children and young adults.
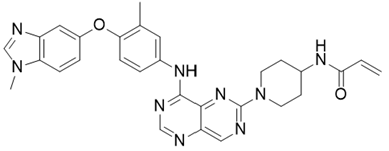
- Drug Name (Brand Name): Hernexeos
Generic Name: zongertinib
Structure:
Approval Date: 8/8/2025 (US), 8/29/2025 (China)
Indication: NSCLC, HER2 mutant/metastatic
Modality: Small molecule
MOA/Target: HER2 (ERBB2) kinase inhibitor
IUPAC Name: N-[1-[4-[3-methyl-4-(1-methylbenzimidazol-5-yl)oxyanilino]pyrimido[5,4-d]pyrimidin-6-yl]piperidin-4-yl]prop-2-enamide
SMILES: CC1=C(C=CC(=C1)NC2=NC=NC3=CN=C(N=C32)N4CCC(CC4)NC(=O)C=C)OC5=CC6=C(C=C5)N(C=N6)C
Sponsor: Boehringer Ingelheim
Significance: Marks Boehringer Ingelheim’s first U.S. oncology drug approval and introduces the first oral HER2-selective TKI for NSCLC—offering a convenient, tolerable alternative to Enhertu, the HER2-targeting ADC from AstraZeneca and Daiichi-Sankyo.
- Drug Name (Brand Name): BRINSUPRI
Generic Name: brensocatib
Structure:
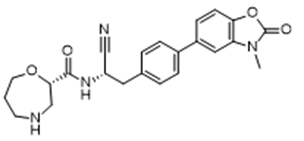
Approval Date: 8/12/2025
Indication: Non-cystic fibrosis bronchiectasis (NCFB)
Modality: Small molecule
MOA/Target: Reversible inhibitor of dipeptidyl peptidase 1 (DPP1)
IUPAC Name: (2S)-N-[(1S)-1-cyano-2-[4-(3-methyl-2-oxo-1,3-benzoxazol-5-yl)phenyl]ethyl]-1,4-oxazepane-2-carboxamide
SMILES: CN1C2=C(C=CC(=C2)C3=CC=C(C=C3)CC@@HNC(=O)[C@@H]4CNCCCO4)OC1=O
Sponsor: Insmed
Significance: First FDA-approved therapy for non-cystic fibrosis bronchiectasis and first DPP1 inhibitor for neutrophil-mediated diseases, reducing exacerbations by 21% (10 mg) and 20% (25 mg) in the phase 3 ASPEN trial, addressing neutrophilic inflammation, a root cause of NCFB progression (NEJM, 2025; DOI: 10.1056/NEJMoa2411664).
26. Drug Name (Brand Name): DAWNZERA
Generic Name: donidalorsen
Approval Date: 8/21/2025
Indication: Prophylaxis to prevent attacks of hereditary angioedema (HAE) in adults and pediatric patients 12 years and older
Modality: Oligonucleotide (antisense oligonucleotide, ASO)
MOA/Target: RNA-targeted inhibitor of plasma prekallikrein (PKK) mRNA
Sponsor: Ionis Pharmaceuticals
Significance: First and only RNA-targeted prophylactic for HAE, reducing attacks by 81% (Q4W) and 87% (from second dose) in OASIS-HAE trial, with longest dosing interval (Q4W or Q8W).
- Drug Name (Brand Name): Wayrilz
Generic Name: rilzabrutinib
Structure:
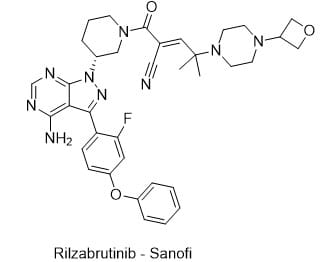
Approval Date: 08/29/2025
Indication: Treatment of adults with persistent or chronic immune thrombocytopenia (ITP) who have had insufficient response to prior therapy
Modality: Small molecule
MOA/Target: Covalent, reversible Bruton’s tyrosine kinase (BTK) inhibitor; reduces B-cell and Fc receptor–mediated autoantibody activity leading to platelet destruction
IUPAC Name: (E)-2-[(3R)-3-[4-amino-3-(2-fluoro-4-phenoxyphenyl)pyrazolo[3,4-d]pyrimidin-1-yl]piperidine-1-carbonyl]-4-methyl-4-[4-(oxetan-3-yl)piperazin-1-yl]pent-2-enenitrile
SMILES: CC(C)(/C=C(\C#N)/C(=O)N1CCC[C@H](C1)N2C3=NC=NC(=C3C(=N2)C4=C(C=C(C=C4)OC5=CC=CC=C5)F)N)N6CCN(CC6)C7COC7
Sponsor: Sanofi (originally developed by Principia Biopharma, acquired 2020)
- Drug Name (Brand Name): Forzinity
Generic Name: elamipretide
Structure:
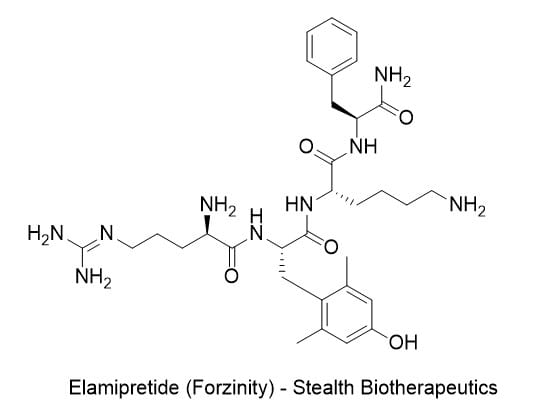
Approval Date:9/19/2025
Indication: Barth syndrome in patients weighing at least 30 kg
Modality: Small molecule
MOA/Target: Cardiolipin stabilizer in inner mitochondrial membrane
IUPAC Name: (2S)-6-amino-2-[(2S)-2-[(2R)-2-amino-5-carbamimidamidopentanamido]-3-(4-hydroxy-2,6-dimethylphenyl)propanamido]-N-[(1S)-1-carbamoyl-2-phenylethyl]hexanamide
SMILES: CC1=CC(=CC(=C1C[C@@H](C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CC2=CC=CC=C2)C(=O)N)NC(=O)[C@@H](CCCN=C(N)N)N)C)O
Sponsor: Stealth Biotherapeutics
Significance: First FDA-approved treatment for Barth syndrome, a rare X-linked mitochondrial disorder primarily affecting males with cardiomyopathy, skeletal myopathy, and growth delays. Accelerated approval via surrogate endpoint of improved knee extensor strength; subcutaneous daily dosing with mild injection-site reactions as primary adverse event. Represents a milestone in mitochondrial medicine for ultra-rare diseases.
29. Drug Name (Brand Name): Keytruda Qlex
Generic Name: pembrolizumab and berahyaluronidase alfa-pmph
Approval Date: 9/19/2025
Indication: Adult and pediatric (12 years and older) solid tumor indications approved for intravenous pembrolizumab
Modality: Combination therapy (monoclonal antibody + enzyme)
MOA/Target: PD-1 inhibitor with hyaluronidase for subcutaneous delivery
Sponsor: Merck
Significance: First subcutaneous formulation of pembrolizumab, reducing administration time to 1–2 minutes compared to 30 minutes for IV Keytruda. Approved for 38 solid tumor indications, with comparable efficacy and safety (MK-3475A-D77 trial), enhancing patient convenience and healthcare efficiency.
30. Drug Name (Brand Name): Inluriyo
Generic Name: imlunestrant
Structure:
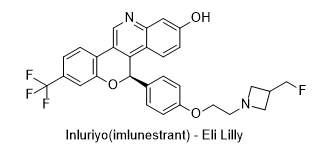
Approval Date: 9/25/2025
Indication: ER-positive, HER2-negative, ESR1-mutated advanced or metastatic breast cancer with disease progression following at least one line of endocrine therapy
Modality: Small molecule
MOA/Target: Selective estrogen receptor degrader (SERD)
IUPAC Name: (7R,13S,17S)-17-amino-13-methyl-7-(1-methyl-1H-pyrazol-4-yl)-6,7,8,9,11,12,13,14,15,16-decahydro-17H-cyclopenta[a]phenanthren-3-ol
SMILES: C1C(CN1CCOC2=CC=C(C=C2)[C@@H]3C4=C5C=CC(=CC5=NC=C4C6=C(O3)C=C(C=C6)C(F)(F)F)O)CF
Sponsor: Eli Lilly
Significance: First oral SERD approved for ESR1-mutated breast cancer, offering a targeted therapy for patients with disease progression after endocrine therapy. Demonstrates significant efficacy in precision oncology for a genetically defined subset of breast cancer patients.
31. Drug Name (Brand Name) : PALSONIFY
Generic Name : paltusotine
Structure :
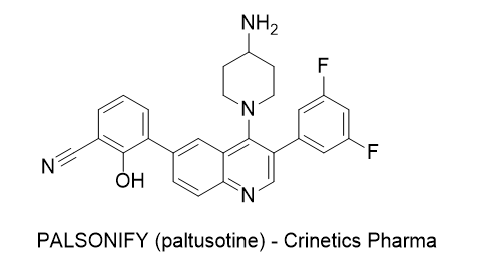
Approval Date : 9/25/2025
Indication : First-line treatment of adults with acromegaly who had an inadequate response to surgery and/or for whom surgery is not an option
Modality : Small molecule
MOA/Target : Selectively-targeted somatostatin receptor type 2 (SST2) agonist
IUPAC Name : 3-[4-(4-aminopiperidin-1-yl)-3-(3,5-difluorophenyl)quinolin-6-yl]-2-hydroxybenzonitrile
SMILES: C1CN(CCC1N)C2=C3C=C(C=CC3=NC=C2C4=CC(=CC(=C4)F)F)C5=CC=CC(=C5O)C#N
Sponsor : Crinetics Pharmaceuticals
Significance : First once-daily oral therapy for acromegaly, with rapid, durable IGF-1 control and reduced symptom burden in PATHFNDR-1/2 trials; well-tolerated with no serious adverse events in randomized portions.
32. Drug Name (Brand Name) : Rhapsido
Generic Name : remibrutinib
Structure :
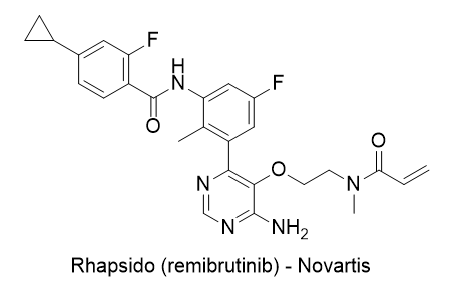
Approval Date : 9/30/2025
Indication : Chronic Spontaneous Urticaria (CSU)
Modality : Small molecule
MOA/Target : Oral Bruton's Tyrosine Kinase (BTK) inhibitor
IUPAC Name : N-[3-[6-amino-5-[2-[methyl(prop-2-enoyl)amino]ethoxy]pyrimidin-4-yl]-5-fluoro-2-methylphenyl]-4-cyclopropyl-2-fluorobenzamide
SMILES: CC1=C(C=C(C=C1NC(=O)C2=C(C=C(C=C2)C3CC3)F)F)C4=C(C(=NC=N4)N)OCCN(C)C(=O)C=C
Sponsor : Novartis
Significance : The first oral targeted BTK inhibitor approved globally for CSU. Classified as a first-in-class drug. Notably, its China NDA was accepted with priority review on March 6, 2025. This approval underscores Novartis’ leadership in immunology and inflammation.