DPP1 Inhibitors: First FDA Approval — China’s Fast-Follow Pipeline
Brensocatib earns FDA approval for NCFB, marking the first validated DPP1 inhibitor. We review the global and China pipelines, including HSK31858 and XH-S004.
Why This Matters
Dipeptidyl peptidase 1 (DPP1, also called cathepsin C) activates neutrophil serine proteases (NE, PR3, CatG), driving inflammation in diseases such as non-cystic fibrosis bronchiectasis (NCFB), COPD, and asthma. Inhibiting DPP1 reduces protease-mediated tissue damage without broadly suppressing immunity. With the FDA approval of brensocatib, DPP1 inhibitors have moved from a promising concept to clinically validated therapy—a pivotal moment for global and Chinese respiratory pipelines.
Mechanistic Rationale
Target Biology
- DPP1 is a lysosomal cysteine protease that activates pro-neutrophil serine proteases (NSPs) during neutrophil maturation in the bone marrow and, to a lesser degree, on extracellular surfaces.
- NSP activation drives tissue damage, mucus hypersecretion, and exacerbations in airway diseases.
- Clinical observations in Papillon–Lefèvre syndrome (PLS, DPP1 loss-of-function) show reduced NETosis but preserved bacterial killing, supporting a favorable therapeutic window.
- Animal models confirm that DPP1 inhibition reduces inflammation without impairing neutrophil recruitment.
Biomarker-Guided Development
- Clinical programs measure sputum or blood NE, PR3, and CatG activity alongside exacerbation endpoints.
- Full NSP suppression correlates with stronger benefits, but partial inhibition is still clinically effective (AIRLEAF, HSK31858).
Design Considerations
Aortic Binding and Medicinal Chemistry
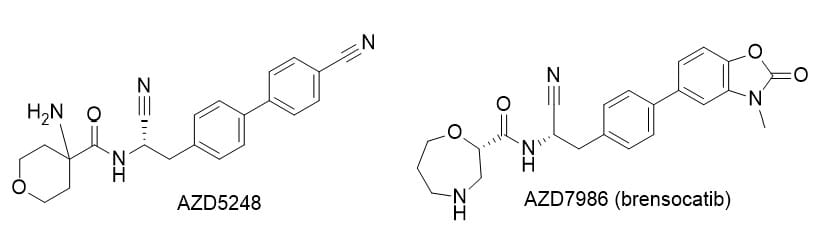
- AstraZeneca’s AZD5248 was discontinued due to persistent aortic binding in rats, likely from covalent reactivity with elastin aldehyde groups.
- Optimization led to AZD7986 (brensocatib), which retains potency while eliminating the vascular retention risk.
Reversible vs Irreversible Inhibition
- Irreversible inhibitors (e.g., GSK2793660) caused mucocutaneous adverse events.
- Reversible covalent inhibitors (brensocatib, HSK31858, BI 1291583) allow dose titration, balancing efficacy and tolerability.
Warhead Chemistry
- Most DPP1 inhibitors are nitrile-based dipeptidyl compounds forming reversible thioimidate bonds with the active-site cysteine.
Key Milestones: FDA Approval & Clinical Validation
| Date | Event |
|---|---|
| Aug 12 2025 | FDA approves BRINSUPRI™ (brensocatib) for NCFB (ages ≥12, 10 mg & 25 mg oral doses). |
| 2025 | BI 1291583 (Boehringer) Phase 2 AIRLEAF reports ~30% exacerbation reduction (ERJ, Jan 2025). |
| 2025 | HSK31858 (Haisco) Phase 2 reports 48–59% NCFB exacerbation reduction, decreased sputum volume (Lancet Respiratory Medicine, Mar 2025). |
| 2025 | HSK31858 launches Phase III pivotal trial in China (CTR20243623; ongoing, recruiting) and initiates long-term safety extension study (CTR20253262; not yet recruiting). Receives Breakthrough Therapy Designation from CDE. |
| 2025 | XH S004 (Fosun) Phase II in China for NCFB; licensed to Expedition Therapeutics for global development in a deal worth up to $645 M. |
DPP1 Inhibitor Pipeline & Clinical Highlight
| Drug (Other Names) | Company | Indications | Status (as of Aug 12 2025) |
|---|---|---|---|
| Brensocatib (BRINSUPRI™, INS1007, AZD7986) | Insmed | NCFB | FDA-approved – first & only NCFB therapy. |
| HS | Phase 2 (CEDAR; NCT06685835) recruiting. | ||
| CRSsNP | Phase 2b ongoing (NCT06013241). | ||
| HSK31858 | Haisco (licensee: Chiesi ex-China) | NCFB | Phase III pivotal trial in China (CTR20243623; ongoing, recruiting) + long-term safety extension (CTR20253262; initiated Aug 13 2025, not yet recruiting). Breakthrough Therapy Designation by CDE. Phase 2 showed reduced exacerbations. |
| Bronchial asthma | Phase 2 (NCT06637254). | ||
| Airway mucus hypersecretion | Phase 2 ongoing. | ||
| Verducatib (BI-1291583) | Boehringer Ingelheim | Bronchiectasis | Phase 2 (AIRLEAF) results published 2025 (ERJ). Additional trials registered (e.g., AIRTIVITY). |
| XH-S004 | Shanghai Fosun / Expedition Therapeutics | NCFB | Phase II in China for NCFB; licensed globally (ex-China) to Expedition Therapeutics. |
| RSS0343 | Reistone (Jiangsu Hengrui affiliate) | NCFB | Phase 1 completed; Phase 2 underway (NCT06775340). |
| MDI-0151 | Alivexis / Melodia | Neutrophil-mediated diseases | Preclinical. |
| GSK2793660 | GSK | Bronchiectasis / other | Terminated early due to class-related skin/dental AEs with irreversible DPP1 inhibition in healthy volunteers. |
China Focus — Fast Followers
- Haisco (HSK31858): Most advanced domestically; now in Phase III pivotal NCFB study plus a long-term safety extension trial. Also in Phase 2 expansion for asthma and mucus hypersecretion. Ex-China rights with Chiesi.
- Hengrui/Reistone (RSS0343): On track with Phase 2 NCFB.
- Fosun (XH S004): Phase II in China for NCFB, poised for global development via $645M Expedition deal.
What to Watch Next
- Post-marketing surveillance of brensocatib — safety, real-world efficacy, and reimbursement uptake.
- HSK31858 Phase 3 results and potential NMPA review acceleration.
- Indication expansion:
- Brensocatib: CRSsNP, hidradenitis suppurativa
- HSK31858: asthma, mucus hypersecretion
- BI 1291583: additional respiratory indications
- Long-term safety and dose optimization, balancing NSP suppression and tolerability through reversible inhibition.
Sources
- Insmed Incorporated. FDA Approves BRINSUPRI™ (brensocatib). August 12, 2025.
- Patel N, et al. Therapeutic targeting of DPP1 in inflammatory airway diseases. Frontiers in Immunology, 2023;14:1239151.
- Smith J, et al. Role of DPP1 inhibitors in neutrophil-driven respiratory diseases. European Respiratory Review, 2025;34(176):240257.
- Jones R, et al. Clinical advances in bronchiectasis: the new era of DPP1 inhibitors. Respiratory Medicine, 2025;S0954-6111(25)00241-0.
- SinoDrugWatch. Fosun Pharma bets on DPP1 inhibition with $645M out-licensing deal, 2025.
- SinoDrugWatch. FDA approval of brensocatib signals new era for NCFB treatment, 2025.