Breakthroughs from ACS Fall 2025: 13 First-Time Drug Structure Disclosures Across 6 Major Disease Areas
ACS Fall 2025 revealed 13 first-time clinical candidates across infectious disease, immunology, cardiometabolic, neurodegeneration, and oncology. This post reviews their targets, modalities, and competitive positioning—including China-based pipelines.
At the ACS Fall 2025 National Meeting, thirteen new clinical candidates were unveiled with full structures—a rare window into pharma pipelines. The set spans multiple domains: IID432 (Chagas disease topo II inhibitor), GS-1427 (α4β7 antagonist, IBD), AZD0233 (CX3CR1 antagonist, neuroinflammation), PF-07293893 (AMPKγ3 activator, metabolic disease), [¹¹C]MK-7337 (α-synuclein PET tracer), BMS-986470 and BMS-986463 (protein degraders), LRK-4189 (molecular glue degrader), ETN029 (DLL3 radioligand, SCLC), TYRA-200 (FGFR2 covalent inhibitor), MOMA-341 (WRN helicase covalent inhibitor, MSI-H cancers), AZD5335 (EP300/CBP inhibitor), and AZD9574 (PARP1-selective inhibitor).
These candidates reflect key discovery trends: modality expansion beyond classical inhibition, renewed attention to immune trafficking control, and oncology’s pivot toward synthetic lethality and targeted payload delivery. Together, they exemplify how first-in-class small molecules are being redefined by chemistry-driven innovation [1–2].
Summary Table of Disclosed Programs
Program-by-Program Analyses
The section lists all 13 candidates with IUPAC/SMILES, chemical structure, target/MOA, indication, and development stage. Additional context on rationale, validation, potential, competition, and China activity is summarized in the bullet points.
1) IID432 — Novartis
- IUPAC name: (S)-2-(3-cyano-1H-1,2,4-triazol-1-yl)-N-(1-(2-methylquinolin-6-yl)pyrrolidin-3-yl)acetamide
- SMILES: CC1=NC(C=CC(N2C[C@@H](NC(CN3N=C(C#N)N=C3)=O)CC2)=C4)=C4C=C1
- Chemical Structure
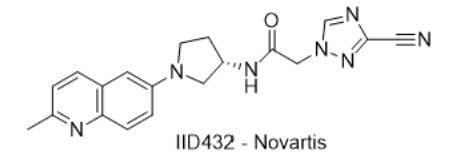
- MOA/Target: T. cruzi topoisomerase II inhibitor
- Indication: Chagas disease
- Why this target? T. cruzi topo II is essential for kinetoplast DNA replication/segregation and sufficiently diverges from human topo II to allow selectivity (Ref 1).
- Validation: Cyanotriazoles act as parasite-selective topo II poisons with rapid cures in mouse models; γH2A assays quantify DNA damage in T. cruzi (Refs 1–2; see also methods in Ref 3).
- Potential: Opportunity to replace/augment benznidazole & nifurtimox (toxicity/limited chronic efficacy) (context in Ref 4).
- Competitive landscape: No approved topo-II-selective trypanocides; DNDi pipelines emphasize nitroimidazoles/oxaboroles (Ref 4).
- China: Limited innovation on T. cruzi topo II; broader antiparasitic peptide efforts active (Ref 4).
- How IID432 compares: Cyanotriazole chemotype prioritizes parasite selectivity and intracellular amastigote cidal activity; first-in-human targeted for 2026 (Ref 1; ACS session listing).
2) BMS-986470 — Bristol Myers Squibb
· IUPAC name: (S)-2-(2,6-dioxopiperidin-3-yl)-4-((1-methyl-6-(2-methylpyridin-4-yl)-2-oxo-1,2,3,4-tetrahydroquinolin-7-yl)amino)isoindoline-1,3-dione
· SMILES: O=C1CCC2=C(C=C(NC3=CC=CC(C(N4[C@H]5CCC(NC5=O)=O)=O)=C3C4=O)C(C6=CC(C)=NC=C6)=C2)N1C
· Chemical Structure
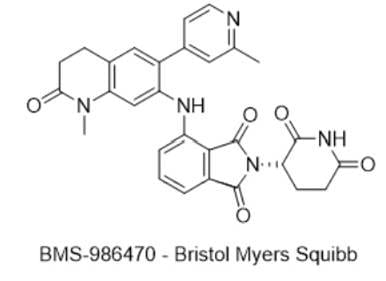
- MOA/Target: Dual ZBTB7A/WIZ molecular glue degrader (CRBN-recruiting)
- Indication: Sickle cell disease
- Development stage: Phase 1 (NCT06481306).
- Why this target? ZBTB7A & WIZ repress fetal hemoglobin (HbF); dual degradation derepresses HbF (Refs 5–6).
- Validation: Preclinical dual-glue data and ASH abstracts demonstrate HbF induction; glue strategy complements/alternatives to gene editing (Refs 5–7).
- Potential: Orally dosed HbF induction could broaden access vs ex vivo editing.
- Competitive landscape: Hydroxyurea (indirect HbF inducer) is standard; competitors include other HbF-pathway glues and genome editors (Refs 6–7).
- China: Early Wee1/TPD activity is strong; SCD-specific dual ZBTB7A/WIZ programs less visible (Ref 8).
- How BMS-986470 compares: First clinical dual ZBTB7A/WIZ glue with patient trial underway (NCT06481306).
3) GS-1427 — Gilead Sciences
· IUPAC name: ethyl (2S)-2-(2-fluoro-6-methyl-4-(3-(trifluoromethyl)morpholino)benzamido)-3-(8-(1-methyl-2,4-dioxo-1,4 dihydropyrido[3,4-d]pyrimidin-3(2H)-yl)quinolin-5-yl)propanoate
· SMILES: CN(C(N1C2=C(N=CC=C3)C3=C(CC@HC(OCC)=O)C=C2)=O)C6=CN=CC=C6C1=O
· Chemical structure
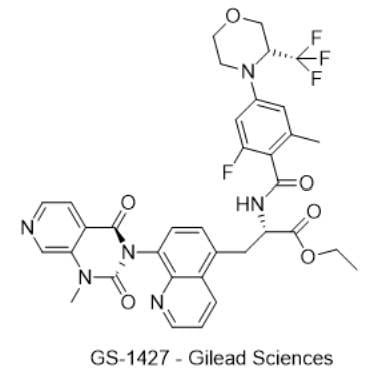
- MOA/Target: α4β7 integrin inhibitor (oral small molecule)
- Indication: Inflammatory bowel disease (IBD)
- Development stage: Phase 2 (NCT06290934).
- Why this target? α4β7 controls gut-homing lymphocyte trafficking—validated gut-selective immunomodulation pathway (vedolizumab precedent) (Refs 9–10).
- Validation: Clinical success of vedolizumab; trial experience with oral antagonists (Ref 10; competitive MORF-057 context).
- Potential: Oral “vedolizumab-like” effect with improved convenience and possibly cost.
- Competitive landscape: Vedolizumab (approved mAb); MORF-057 in late stage (Ref 10; pipeline reports).
- China: α4β7 work includes biosimilars/antagonist research footprints (Ref 10).
- How GS-1427 compares: Differentiates via oral modality aiming for gut selectivity akin to biologics (Refs 9–10).
4) PF-07899895 — Pfizer
- IUPAC name: 3-(3-((1-aminocyclohexyl)methoxy)-4-cyano-5-(methylthio)phenyl)imidazo[1,2-a]pyridine-5-carbonitrile
- SMILES: NC1(COC2=C(C#N)C(SC)=CC(C3=CN=C4N3C(C#N)=CC=C4)=C2)CCCCC1
- Chemical Structure
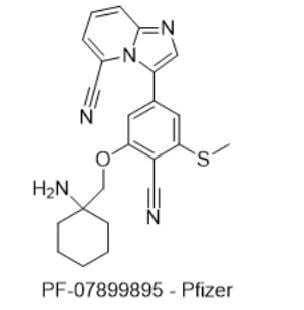
- MOA/Target: Colon-targeted pan-SIK1–3 inhibitor
- Indication: IBD
- Development stage: Phase 1 (NCT06137729).
- Why this target? SIK inhibition skews macrophages toward IL-10 and anti-inflammatory phenotypes (Refs 11–12).
- Validation: Multiple chemical series show SIK2/3 blockade increases IL-10; early patient signals from class precedents (Refs 11–12).
- Potential: Broad immunomodulation with local colon delivery to minimize systemic risks.
- Competitive landscape: Galapagos/Nimbus/Sitryx SIK programs pre-to-mid stages (Ref 11).
- China: Patent activity reported; no disclosed clinical SIK programs specific to IBD (Ref 13).
- How PF-07899895 compares: Designed for colon targeting, potentially improving therapeutic index vs systemic SIK inhibitors (Refs 11–12).
5) AZD0233 — AstraZeneca
- IUPAC name: N-(4-((R)-2-(4-fluoro-6-methoxypyridin-3-yl)propyl)-6-(((R)-1-hydroxy-4-methylpentan-2-yl)amino)-1,3,5-triazin-2-yl)methanesulfonamide
- SMILES: OCC@@HNC1=NC(CC@@HC2=C(F)C=C(OC)N=C2)=NC(NS(C)(=O)=O)=N1
- Chemical Structure
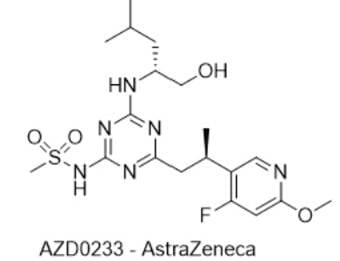
- MOA/Target: CX3CR1 (fractalkine receptor) antagonist
- Indication: Cardiovascular disease (atherosclerosis/inflammation)
- Development stage: Phase 1 (NCT06381466).
- Why this target? CX3CR1 mediates monocyte recruitment; genetic/experimental evidence links axis to atherosclerosis and MI injury (Refs 14–16).
- Validation: Prior AZD8797/KAND567 data in animals and Phase 2a STEMI safety with signals of cardioprotection (FRACTAL) (Refs 15–16).
- Potential: Anti-inflammatory stabilization of plaque and microvascular protection beyond lipid lowering.
- Competitive landscape: Kancera’s KAND567 in Phase 2a (STEMI; other indications) (Refs 15–16).
- China: Early antibody licensing noted; small-molecule CX3CR1 programs not yet prominent (industry tracking; Ref 17).
- How AZD0233 compares: Next-gen small-molecule antagonist aiming for oral potency with improved selectivity over legacy CX3CR1 tools (Refs 14–16).
6) PF-07293893 — Pfizer
- IUPAC name: (R)-4-(3-chloro-2-fluorophenyl)-5-fluoro-2-(4-fluoro-1-methyl-1H-pyrazol-3-yl)-6-((1-((1R,2S)-2-fluorocyclopropane-1-carbonyl)azetidin-3-yl)amino)-4-methyl-3,4-dihydro-2,7-naphthyridin-1(2H)-one
· SMILES: O=C1N(C2=NN(C)C=C2F)CC@(C3=CC=CC(Cl)=C3F)C4=C(F)C(NC5CN(C([C@H]6C[C@@H]6F)=O)C5)=NC=C41
· Chemical Structure
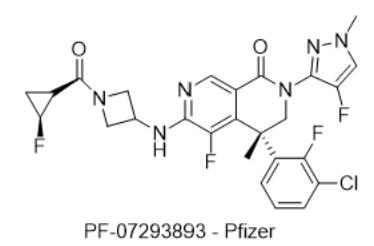
- MOA/Target: AMPKγ3 activator
- Indication: Heart failure
- Development stage: Phase 1 completed (NCT06177457).
- Why this target? AMPKγ3 activation enhances cardiac energetics and fatty-acid oxidation—relevant to HFpEF/HFrEF metabolic deficits (Refs 18–19).
- Validation: Direct small-molecule AMPK activators (ADaM-site) show robust metabolic effects; γ-subunit selectivity may limit off-target effects (Ref 19).
- Potential: First γ3-selective approach for cardiac energetic rescue.
- Competitive landscape: Indirect activators (e.g., metformin) lack cardiac selectivity; few γ3-specific programs disclosed (Refs 18–20).
- China: Broader AMPK work in diabetes/metabolism; γ3-specific HF programs not prominent.
- How PF-07293893 compares: Selectivity for γ3 may expand therapeutic window vs pan-AMPK activators (Refs 18–20).
7) [¹¹C]MK-7337 — Merck & Co.
· IUPAC name: (R,E)-5-(2-(6-(1H-imidazol-1-yl)pyridin-3-yl)vinyl)-2-(3-((methoxy-11C)methyl)-4-(pyrimidin-2-yl)piperazin-1-yl)pyrimidine
· SMILES: [11CH3]OC[C@H](N(C1=NC=CC=N1)CC2)CN2C(N=C3)=NC=C3/C=C/C(C=N4)=CC=C4N5C=CN=C5
· Chemical Structure
![chemical structure of [¹¹C]MK-7337 — Merck - pet ligand radiotracer - prakinson desease imaging](https://www.sinodrugwatch.com/content/images/2025/08/data-src-image-a21560ac-e6ed-469d-b517-843e636dde8f.png)
- MOA/Target: α-Synuclein PET ligand (radiotracer)
- Indication: Parkinson’s disease imaging
- Development stage: Preclinical/early translational (session + press coverage).
- Why this target? α-Syn aggregates define synucleinopathies; non-invasive imaging enables patient selection and pharmacodynamic readouts (Refs 21–23).
- Validation: Emerging human PET data with alternative tracers (e.g., ACI-12589) demonstrate feasibility (Refs 21–22).
- Potential: Trial enrichment and response tracking for anti-synuclein therapeutics.
- Competitive landscape: AC Immune ACI-12589 (clinical), others in discovery (Refs 21–22).
- China: Academic tracer efforts in preclinical stages (general landscape reviews).
- How [¹¹C]MK-7337 compares: Merck reports high affinity & selectivity and promising brain kinetics; ¹¹C enables early human PoC though limits broad deployment (Ref 23).
8) FOG-001 — Parabilis Medicines
· IUPAC name: 2,2'-((1''S,2R,2'S,5'S,8'S,10''E,12'E,18''S,21''S,27''S,30''S,33''S,42''S,45''S,48''S)-1-acetyl-42''-(((S)-1-amino-1-oxopropan-2-yl)carbamoyl)-45''-(benzo[b]thiophen-3-ylmethyl)-30''-benzyl-21''-(3-carboxybenzyl)-24'',24'',27''-trimethyl-5'-neopentyl-3',5'',6',16',16'',19'',22'',25'',28'',31'',39'',44'',47'',50'',52''-pentadecaoxo-48''-(thiophen-3-ylmethyl)-6''-oxa-1',4',4'',7',17'',20'',23'',26'',29'',32'',38'',43'',46'',49'',51''-pentadecaazadispiro[pyrrolidine-2,15'-cyclohexadecane-8',15''-tricyclo[31.17.2.11,4]tripentacontane]-10'',12'-dien-2',18''-diyl)diacetic acid
· SMILES: O=C(C1=CC=CC(C[C@@H](C(NC(C)(C)C(N[C@@H](C)C(N[C@@H](CC2=CC=CC=C2)C(N[C@@H]3C(N[C@](C(N[C@@H](CC4=CSC=C4)C(N[C@@H](CC5=CSC6=C5C=CC=C6)C(N[C@H](C(N[C@@H](C)C(N)=O)=O)CCC(NCCCC3)=O)=O)=O)=O)(C7)CCN7C(OCCC/C=C/CCC8)=O)=O)=O)=O)=O)=O)NC([C@H](CC(O)=O)NC([C@@]98NC([C@H](CC(C)(C)C)NC([C@H](CC(O)=O)NC([C@]%10(C/C=C/CCC9)CCCN%10C(C)=O)=O)=O)=O)=O)=O)=C1)O
· Chemical Structure
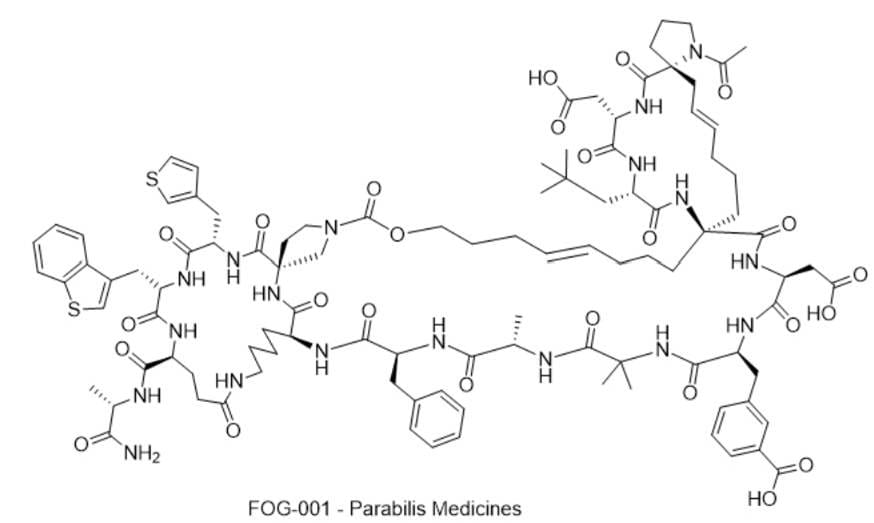
- MOA/Target: β-Catenin/TCF4 interaction inhibitor (stapled peptide)
- Indication: Metastatic solid tumors (Wnt-driven)
- Development stage: Phase 1/2 (NCT05919264).
- Why this target? β-Catenin/TCF4 PPI is the transcriptional engine of canonical Wnt signaling—critical in APC-mutant CRC (Refs 24–26).
- Validation: Genetic suppression and PPI inhibitors block Wnt transcription and tumor growth in models (Refs 24–26).
- Potential: Direct pathway blockade for Wnt-addicted tumors where upstream inhibition fails.
- Competitive landscape: Tegavivint (β-catenin/TCF disruption via TBL1/TBLR1), Porcupine inhibitors, tankyrase inhibitors (Refs 24–26).
- China: Strong peptide manufacturing capabilities; limited public clinical Wnt PPI efforts.
- How FOG-001 compares: Stapled/bicyclic design aims for cell penetration & PPI disruption with an injectable modality (Refs 24–26).
9) TYRA-200 — Tyra Biosciences
· IUPAC name: N-(1-(5-fluoro-2-((1-(2-hydroxy-2-methylpropyl)-1H-pyrazol-4-yl)amino)pyrimidin-4-yl)-3-methyl-1H-indol-5-yl)acrylamide
· SMILES: O=C(NC1=CC(C(C)=CN2C3=NC(NC4=CN(CC(C)(C)O)N=C4)=NC=C3F)=C2C=C1)C=C
· Chemical Structure
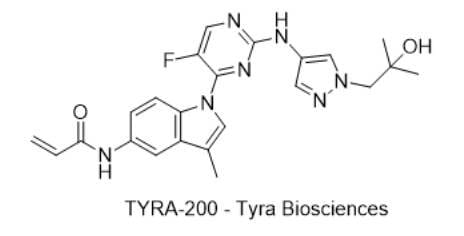
- MOA/Target: Covalent FGFR2 inhibitor (mutant/fusion-selected)
- Indication: Cholangiocarcinoma (FGFR2 fusions/mutations)
- Development stage: Phase 1 (NCT06160752).
- Why this target? FGFR2 fusions drive ~10–15% ICC; on-target dependency validated clinically (Refs 27–28).
- Validation: Multiple approved FGFR inhibitors demonstrate tumor control; resistance mutations (gatekeeper/activation loop) emerge (Ref 28).
- Potential: Covalent design to cover resistant FGFR2 variants, prolonging benefit.
- Competitive landscape (approved): Pemigatinib, futibatinib; (pipeline) RLY-4008, infigratinib re-reads, etc. (Refs 27–28).
- China: Pemigatinib approved/used; domestic pan-FGFR programs active (Refs 29–30).
- How TYRA-200 compares: Structure-guided covalent chemistry aimed at mutant-selective coverage vs reversible agents (Refs 27–28).
10) MOMA-341 — Moma Therapeutics
· IUPAC name: (R)-1-(5-(4-amino-5-fluoro-6-(trifluoromethyl)nicotinoyl)-2-(4-cyclopropyl-2-hydroxyphenyl)-2,3,4,5,5a,6,8,9-octahydro-7H-1,2,5,7-tetraazabenzo[cd]azulen-7-yl)prop-2-en-1-one
· SMILES: OC1=CC(C2CC2)=CC=C1N3N=C4CCN(C(C=C)=O)C[C@H]5C4=C3CCN5C(C6=CN=C(C(F)(F)F)C(F)=C6N)=O
· Chemical structure
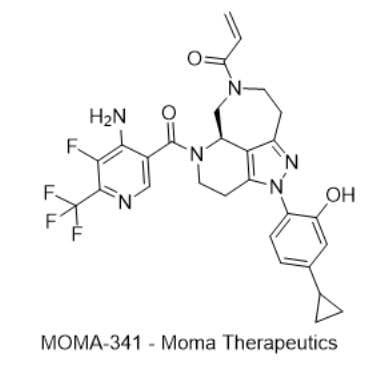
- MOA/Target: Covalent WRN helicase inhibitor
- Indication: Advanced/metastatic solid tumors (MSI-H focus)
- Development stage: Phase 1 (NCT06974110).
- Why this target? WRN is synthetic-lethal in MSI-H cancers (CRISPR screens) (Ref 31).
- Validation: Independent discovery campaigns and first-in-human WRN programs (e.g., HRO761) support target dependency (Refs 31–32).
- Potential: Precision therapy beyond immunotherapy for MSI-H tumors.
- Competitive landscape: Novartis HRO761; other biotech entries disclosed (Ref 31).
- China: Patent-stage WRN efforts reported (Refs 33–34).
- How MOMA-341 compares: Covalent design with broad MSI-H activity profile; clinical PoC underway (NCT06974110).
11) ETN029 — Mariana Oncology (acquired by Novartis)
· IUPAC name: 2,2',2''-(10-(2-((4-((5R,8S,11S,14S,17S,20S,23R)-23-((2S)-2-((3R)-2-((S)-2-acetamido-3-(1H-indol-3-yl)propanamido)-3-hydroxybutanamido)-4,4-dimethylpentanamido)-17-(2-amino-2-oxoethyl)-5-(((S)-1-((S)-2-carbamoylpyrrolidin-1-yl)-3-(1H-indol-3-yl)-1-oxopropan-2-yl)carbamoyl)-8-(carboxymethyl)-20-((1-(carboxymethyl)piperidin-4-yl)methyl)-14-(naphthalen-2-ylmethyl)-7,10,13,16,19,22-hexaoxo-1,3-dithia-6,9,12,15,18,21-hexaazacyclotetracosan-11-yl)butyl)amino)-2-oxoethyl)-1,4,7,10-tetraazacyclododecane-1,4,7-triyl)triacetic acid
· SMILES: OC(CN1CCN(CC(O)=O)CCN(CC(O)=O)CCN(CC(NCCCC[C@H]2C(N[C@H](C(N[C@@H](CSCSC[C@@H](C(N[C@H](C(N[C@H](C(N[C@H](C(N2)=O)CC3=CC4=C(C=C3)C=CC=C4)=O)CC(N)=O)=O)CC5CCN(CC(O)=O)CC5)=O)NC([C@@H](NC(C(NC([C@@H](NC(C)=O)CC6=CNC7=C6C=CC=C7)=O)[C@H](O)C)=O)CC(C)(C)C)=O)C(N[C@H](C(N8[C@@H](CCC8)C(N)=O)=O)CC9=CNC%10=C9C=CC=C%10)=O)=O)CC(O)=O)=O)=O)CC1)=O
· Chemical Structure
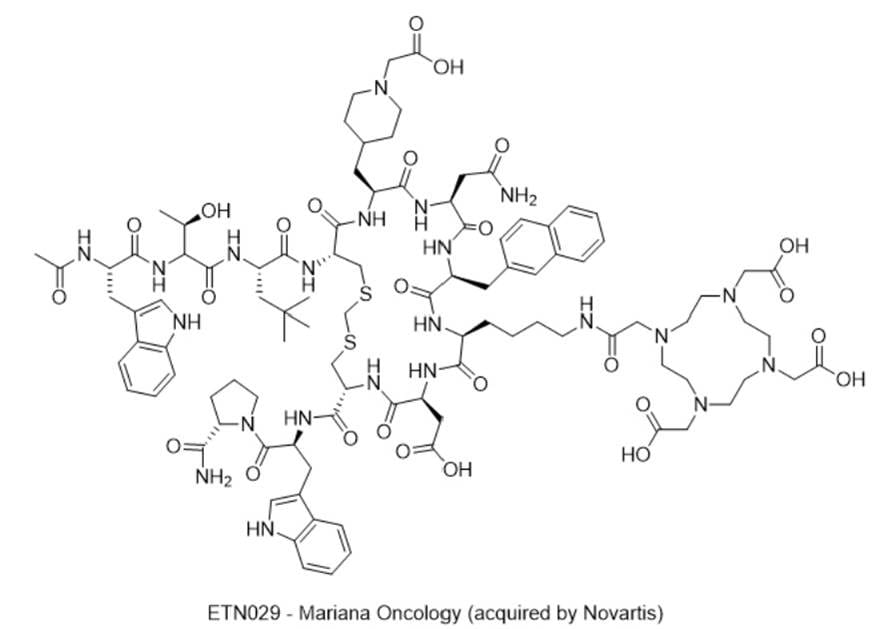
- MOA/Target: DLL3-targeted radioligand therapy (DOTA-chelated; α-emitter strategy)
- Indications: SCLC; neuroendocrine prostate cancer
- Development stage: Phase 1 (NCT07006727).
- Why this target? DLL3 is highly expressed in SCLC/NEPC with minimal normal tissue expression—ideal for targeted delivery (Refs 35–36).
- Validation: Clinical success of DLL3 T-cell engagers and evolving ADCs; radioligands bring potent bystander kill (Refs 35–36).
- Potential: Deep responses in DLL3-high tumors; combination with DNA-damage agents.
- Competitive landscape: Tarlatamab (DLL3 BiTE) approved (context); multiple DLL3 ADCs/TCEs (Refs 35–38).
- China: Zai Lab and others pursuing DLL3 ADCs; active partnering/licensing ecosystem (Refs 37–38).
- How ETN029 compares: α-particle approach (e.g., 225Ac) may exceed ADC potency with short path length; early safety/PK readouts in NCT07006727 (Ref 36).
12) LRK-4189 — Larkspur Biosciences
· IUPAC name: (1R,4S)-4-(4-(4-(((S)-2,6-dioxopiperidin-3-yl)amino)-2-fluorophenyl)piperazin-1-yl)-N-((1s,4S)-4-((5-fluoro-4-(3-(2-oxopyridin-1(2H)-yl)phenyl)pyrimidin-2-yl)amino)cyclohexyl)cyclohexane-1-carboxamide
· SMILES: O=C1CC[C@@H](C(N1)=O)NC2=CC=C(N3CCN(CC3)[C@H]4CC[C@@H](C(N[C@H]5CC[C@@H](NC6=NC(C7=CC=CC(N8C=CC=CC8=O)=C7)=C(F)C=N6)CC5)=O)CC4)C(F)=C2
· Chemical Structure
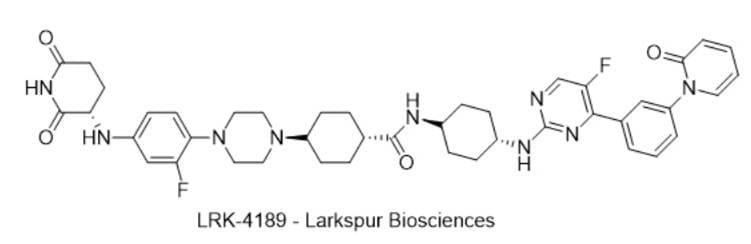
- MOA/Target: PIP4K2C degrader (CRBN-recruiting)
- Indication: MSS colorectal cancer (immune-cold tumors)
- Development stage: Phase 1 planned Q4-2025 (company disclosure).
- Why this target? PIP4K2C supports tumor-intrinsic immune evasion; degradation remodels T-cell infiltration (Ref 39).
- Validation: Preclinical TME reprogramming and T-cell effects in tumor models; early translational data at AACR/JITC (Refs 39–41).
- Potential: First-in-class lipid kinase degrader for MSS CRC, a large unmet need.
- Competitive landscape: Limited direct competitors; PI3K-axis inhibitors are not equivalent.
- China: No public PIP4K2C-degrader clinicals yet.
- How LRK-4189 compares: Degradation (vs inhibition) yields sub-nM target clearance and immune activation signals; IND-enabling complete with 2025 FIH timing (Ref 40).
13) BMS-986463 — Bristol Myers Squibb
· IUPAC name: (S)-3-(5-(((S)-1-((8-fluoro-2-(tetrahydro-2H-pyran-4-yl)quinolin-6-yl)methyl)pyrrolidin-3-yl)oxy)-1-oxoisoindolin-2-yl)piperidine-2,6-dione
· SMILES: FC1=C(N=C(C2CCOCC2)C=C3)C3=CC(CN4C[C@@H](OC5=CC=C(C(N([C@H]6CCC(NC6=O)=O)C7)=O)C7=C5)CC4)=C1
· Chemical Structure
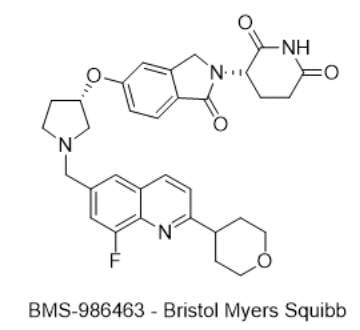
- MOA/Target: WEE1 molecular glue degrader (CRBN-dependent)
- Indication: Advanced solid tumors
- Development stage: Phase 1 (NCT06476808).
- Why this target? WEE1 guards G2/M; loss amplifies replication stress—a vulnerability in TP53-mutant tumors (Refs 42–43).
- Validation: CRBN-dependent WEE1 glues degrade the kinase and outperform inhibitors preclinically; adavosertib limitations motivate new modality (Refs 42–44).
- Potential: Wider therapeutic window via event-driven pharmacology.
- Competitive landscape: Classic WEE1 inhibitors (adavosertib) and new glues from multiple groups (Refs 42–44).
- China: Active TPD scene (e.g., Degron, Neox) working on WEE1/other glues (industry reports; Ref 45).
- How BMS-986463 compares: First clinical WEE1 glue from big pharma; NCT06476808 focuses on dose, PK/PD, and early efficacy.
Discussion
The disclosures highlight how structural transparency clarifies strategic directions across six therapeutic areas:
Neglected infectious disease: IID432 validates parasite-selective topo II poisoning, offering curative potential beyond nitroaromatics (benznidazole/nifurtimox) [3–5].
Immunology: GS-1427 (α4β7) and AZD0233 (CX3CR1) show the move toward precise leukocyte trafficking control in IBD and cardiovascular inflammation [6–10].
Cardiometabolic: PF-07293893 revives interest in AMPK activation, a pathway once hampered by tool compound limitations [11–13].
Neuroscience: [¹¹C]MK-7337 underscores alignment of PET imaging with α-synuclein drug development, supporting disease-modifying trials in Parkinson’s disease [14–16].
Oncology: Protein degraders (BMS-986470, BMS-986463, LRK-4189) [17–19], covalents (TYRA-200, MOMA-341) [20–23], and radioligands (ETN029) [24–26] reveal how modality choice—degradation, covalent locking, and radiotherapy—shapes tumor strategy. Cross-cutting synthetic lethality and resistance-proofing approaches are emerging.
Cross-cutting themes:
- Beyond inhibition – degraders, glues, covalents, and radioligands dominate [3–26].
- Immune cell “traffic control” – gut-homing and chemokine antagonists re-emerge in autoimmunity [6–10].
- China’s intersection – DLL3-targeting drugs (ETN029, ADCs), WRN inhibitors, and FGFR2 covalents overlap with active Chinese pipelines, ensuring global competitive tension [20,24,29–30].
Together, these candidates illustrate how ACS disclosures are now benchmarks of frontier medicinal chemistry, where modality, selectivity, and competitive positioning matter as much as target novelty [1–26].
References
General & News Context
- C&EN. Structures of 13 drug candidates unveiled at ACS Fall 2025. 2025. [Online] (accessed Aug 24, 2025).
- DrugHunter. ACS Fall 2025 First-Time Disclosures. 2025. [Online] (accessed Aug 24, 2025).
IID432 / T. cruzi Topoisomerase II (Chagas disease)
3. Rao, S. P. S.; et al. Cyanotriazoles are selective topoisomerase II poisons that rapidly cure trypanosome infections. Science 2023, 380 (6652), 1349–1356.
4. Ali, M. A.; et al. Imaging assays to detect DNA damage in trypanosome parasites. Bio-Protocol 2024, 14, e500792.
5. Gonzáles-Córdova, R. A.; et al. Trypanosoma cruzi infection induces DNA double-strand breaks and activates DDR in host cells. Infect. Immun. 2024, 92, exxxx.
BMS-986470 / ZBTB7A & WIZ Glues (SCD)
6. ASH 2024 Abstracts on dual HbF-activating glues (ZBTB7A/WIZ).
7. C&EN. Dueling glue degraders take on sickle cell disease. 2024.
8. ClinicalTrials.gov. NCT06481306. BMS-986470 in SCD.
9. BioWorld/industry briefs on China TPD pipelines (Degron, Neox).
GS-1427 / α4β7 (IBD)
10. Feagan, B. G.; et al. Vedolizumab for ulcerative colitis. N. Engl. J. Med. (PMC summary).
11. Morphic press & trial listings for MORF-057.
12. ClinicalTrials.gov. NCT06290934. GS-1427 in IBD.
PF-07899895 / SIK (IBD)
13. Mateer, T.; et al. Dual SIK2/3 inhibitors and anti-inflammatory profiles. ACS Med. Chem. Lett. 2024.
14. PNAS 2023 SIK1/2 selectivity paper.
15. ClinicalTrials.gov. NCT06137729. PF-07899895.
16. BioWorld note on Roche SIK IP (landscape).
AZD0233 / CX3CR1 (CVD)
17. Corona, J. C.; et al. Fractalkine/CX3CR1 in vascular disease. Front. Cardiovasc. Med. 2023, 10, 120xxxx.
18. Kancera. FRACTAL Phase 2a top-line (STEMI). Press + Newcastle Univ. news.
19. Lundberg, P.; et al. AZD8797/KAND567 pharmacology & mechanism. J. Neuroimmunol. / Front. Neuroimmunol..
20. ClinicalTrials.gov. NCT06381466. AZD0233 First-in-Human.
PF-07293893 / AMPKγ3 (Heart Failure)
21. Maack, C.; et al. AMPK as a therapeutic target in HF. Front. Physiol. 2018.
22. Scott, J. W.; et al. ADaM-site activators of AMPK. Nat. Commun. 2021, 12, 190.
23. ClinicalTrials.gov. NCT06177457. PF-07293893 FIH (completed).
[¹¹C]MK-7337 / α-Syn PET (Parkinson’s disease imaging)
24. Villemagne, V. L.; et al. α-Syn PET review. Gen. Psychiatry 2025 (ahead of print).
25. Parkinson’s News Today: human imaging with α-syn tracer(s).
26. ACS Fall 2025 Digitell: Discovery of [¹¹C]MK-7337 (Merck session).
FOG-001 / β-Catenin–TCF (Oncology)
27. Grossmann, T. N.; et al. Direct targeting of β-catenin. PNAS 2012, 109, 17942–17947.
28. Daugherty, R. L.; et al. Targeting β-catenin in Wnt signaling (review). Cancers 2021, 13, 1797.
29. ClinicalTrials.gov. NCT05919264. FOG-001.
TYRA-200 / FGFR2 (Cholangiocarcinoma)
30. Abou-Alfa, G. K.; et al. FGFR2 fusions in ICC and responses to FGFR inhibition. Eur. J. Cancer 2022.
31. Goyal, L.; et al. Resistance to FGFR inhibitors (landscape). Cancer Discov. 2023, 13, 2260–2279.
32. ClinicalTrials.gov. NCT06160752. TYRA-200.
33. Innovent press; China pemigatinib experience.
34. Zhang, L.; et al. Pemigatinib in Chinese patients. Cancer Med. 2023, 12, 1469–1479.
MOMA-341 / WRN (Oncology, MSI-H tumors)
35. Schenone, M.; et al. Discovery of WRN inhibitor HRO761 with MSI-H synthetic lethality. Nature 2024, 631, 123–130.
36. Med. Chem. Lett./Bioorg. Med. Chem. reports of novel WRN inhibitors (2025).
37. BioWorld notes on Jemincare WRN programs.
38. BioWorld notes on Qilu WRN programs.
39. ClinicalTrials.gov. NCT06974110. MOMA-341.
ETN029 / DLL3 Radioligand Therapy
40. ACS Fall 2025 Digitell: Discovery & development of DLL3 RLT.
41. ClinicalTrials.gov. NCT07006727. [225Ac]ETN029 in SCLC/NEPC.
42. Zai Lab ASCO 2025 update on DLL3 ADC ZL-1310.
43. China IP Today: DLL3 ADC deal flow (landscape).
LRK-4189 / PIP4K2C Degrader
44. AACR Cancer Research Abstract 3963: tumor-intrinsic impact of PIP4K2C degradation.
45. Larkspur press: LRK-4189 first-in-class PIP4K2C degrader; FIH Q4-2025.
46. JITC supplement: targeted protein degradation of PIP4K2C.
BMS-986463 / WEE1 Glue
47. Pettersson, M.; et al. Discovery of CRBN-dependent WEE1 molecular glue degraders. Nat. Chem. Biol. 2025, 21, 123–133.
48. Preprint: CRBN-dependent WEE1 glues. BioRxiv 2024.
49. ACS Fall 2025 Digitell: first-in-class WEE1 glue session.
50. ClinicalTrials.gov. NCT06476808. BMS-986463.